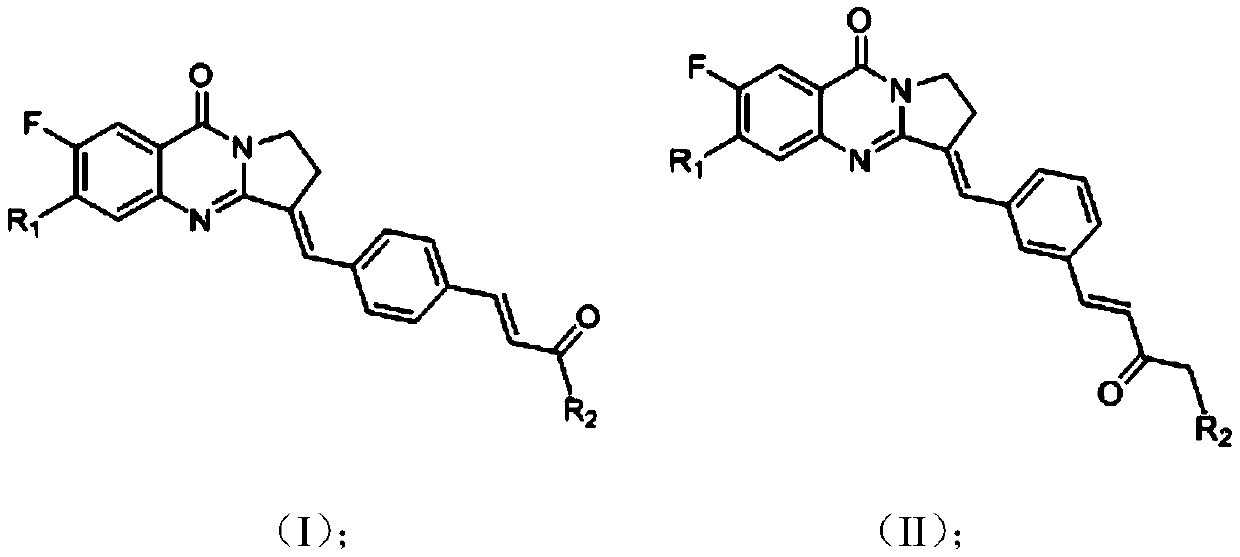
A kind of quinazolinone and α, β-unsaturated ketone conjugate derivative and its preparation method and application
A quinazolinone and unsaturated technology, applied in the field of medicinal chemistry, can solve the problems of high toxicity and side effects, drug resistance, etc., and achieve the effects of inhibiting proliferation, broadly anti-tumor, and inhibiting transcription and expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the synthesis of compound 2
[0046]
[0047] Put 2-amino-4,5-difluorobenzoic acid (5g, 29mmol) and 2-pyrrolidone (5mL) in a 100mL single-necked flask, and add 20mL of phosphorus oxychloride (POCl 3), let it drip for 30min, heat and reflux at 103°C for 24h, add the reaction system dropwise into 200mL ice water, adjust the pH to weak alkalinity with concentrated NaOH solution, and filter with suction to obtain the crude product, which is purified by silica gel column chromatography (elution Reagent: V (petroleum ether): V (ethyl acetate) = 1:1) to obtain compound m1, 5.4 g, yield 85%.
[0048] 1 H NMR (400MHz, CDCl 3 )δ8.20–7.85(m,1H),7.60–7.34(m,1H),4.42–4.06(m,2H),3.19(td,J=7.9,2.7Hz,2H),2.43–2.16(m, 2H).LC-MS m / z:223[M+H] +.
Embodiment 2
[0049] Embodiment 2: the synthesis of compound 3
[0050]
[0051] Take compound 2 (2.22g, 10mmol), diethylaminopropylamine (3ml, 24mmol), put it in a thick-walled pressure bottle, heat and stir at 100°C overnight to obtain an orange-yellow solution, and after the reaction system cools down to room temperature, there are a large amount of light A yellow precipitate was precipitated, filtered with suction, and the filter cake was obtained as a milky white solid, which was vacuum-dried to obtain 2.3 g of a milky white solid, with a yield of 69%.
[0052] 1 H NMR (400MHz, CDCl 3 )δ7.72(d,J=11.7Hz,1H),6.72(s,1H),6.67(d,J=7.7Hz,1H),4.19–4.12(m,2H),3.31(dd,J=10.6 ,5.8Hz,2H),3.11(t,J=7.9Hz,2H),2.63–2.59(m,2H),2.55(q,J=7.1Hz,4H),2.30–2.19(m,2H),1.89 –1.81(m,2H),1.06(t,J=7.1Hz,6H).
Embodiment 3
[0053] Embodiment 3: the synthesis of compound 4
[0054]
[0055] According to the synthetic method of 3, 1.8 g milky white solid was obtained, and the yield was 52%. 1 H NMR (400MHz, CDCl 3 )δ7.67(d,J=11.7Hz,1H),6.62(d,J=7.7Hz,1H),4.13–4.05(m,2H),3.72–3.67(m,2H),3.66–3.61(m ,2H),3.25(dd,J=11.1,5.8Hz,2H),3.04(t,J=7.9Hz,4H),2.51–2.46(m,3H),2.24–2.12(m,4H),1.86– 1.75(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com