Pharmaceutical preparation containing p40 molecule or its gene
A molecular and preparation technology, applied in the field of pharmaceutical preparations, can solve the problems of prone to infection death, weakened anti-bacterial infection, weak anti-infection ability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Production of recombinant IL-23 and p40 molecules
[0043] Total RNA was extracted from the cells of BALB / c mice subcutaneously implanted tumors, and the full-length cDNAs of p19 and p40 were obtained by RT-PCR, and inserted into the cloning site of the animal cell expression vector pcDNA3 (Invitrogen Company) ( Eco RI / Bam HI), and the two cDNAs are connected by an internal ribosomal entry site (IRES) (Duke, GM et al., J. Virol., 66, 1602-1609, 1992). , The IL-23 expression vector is inserted in the downstream of the CMV promoter with the gene arranged in the order of p19 / IRES / p40, and the transcription of p19 and p40 cDNA is controlled by the CMV promoter. In the vector used to express soluble p40 molecules, only p40 cDNA is inserted, and its transcription is controlled by the CMV promoter. The above-mentioned vector was introduced into monkey kidney cells COS-7 (American Type Culture Collection, ATCC) with Lipofectin reagent (Invitrogen Co.), cultured for 48 hours, ...
Embodiment 2
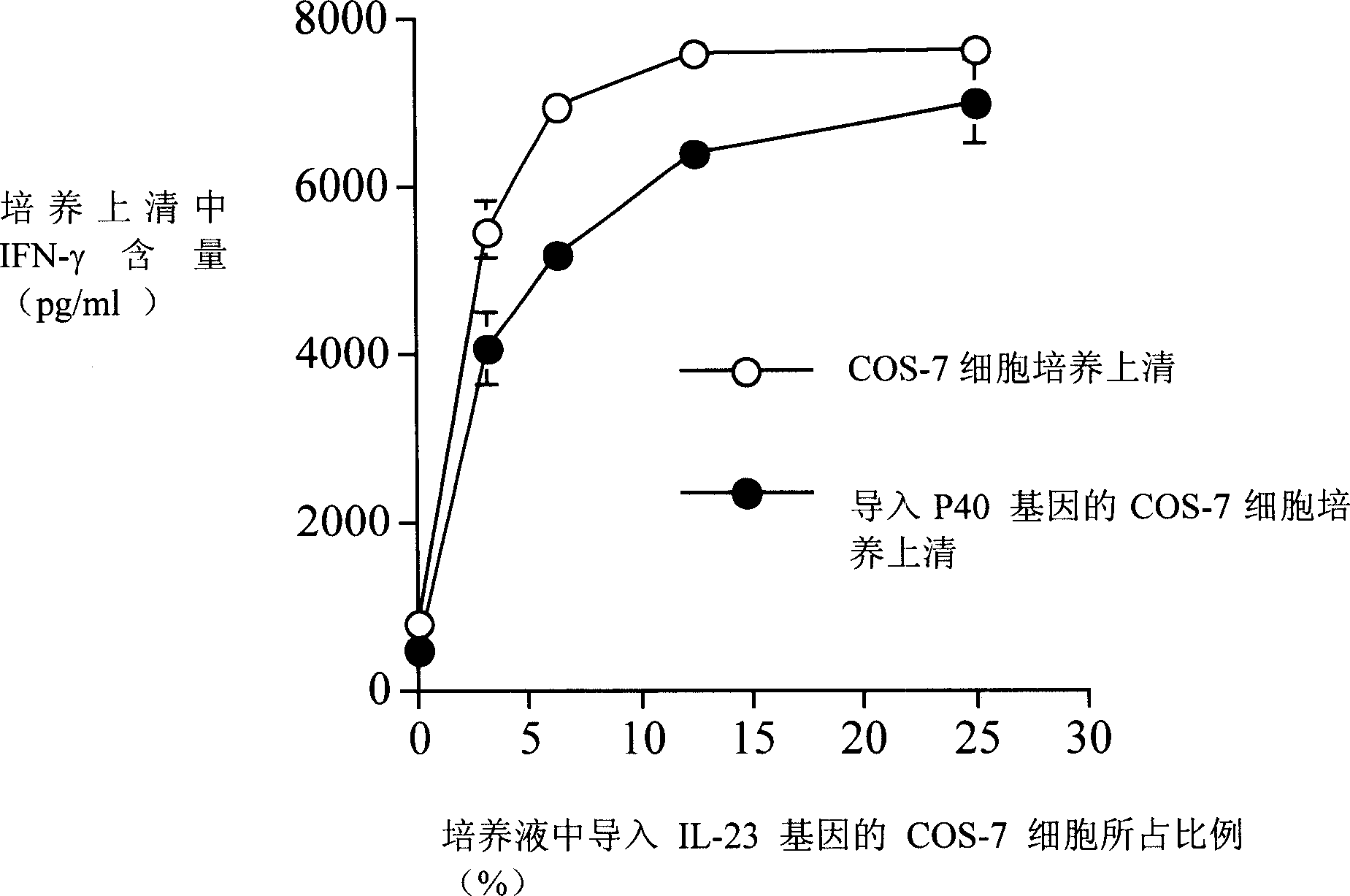
[0046] p40 inhibits IL-23-induced splenocytes to produce IFN-γ
[0047] Spleen cells of C57BL / 6 mice were stimulated with Concanavalin A (5 μg / ml, Sigma Company). After 48 hours, living cells were recovered, rinsed twice with phosphate buffer, and inoculated on 24-well plates (2.5×10 6 per well), adding the culture supernatant of COS-7 cells containing IL-23 for culturing, and adding the culture supernatant of COS-7 cells introduced with p40 gene or the culture supernatant of COS-7 cells without introducing any gene. After culturing for 48 hours, the culture supernatant was recovered, and the content of IFN-γ was determined by ELISA (eBioscience).
[0048] The result is as figure 1shown. When the culture supernatant containing p40 molecule was added, the secretion of IFN-γ was significantly decreased compared with the group added with the culture supernatant not containing p40 molecule. It indicated that p40 molecule significantly inhibited the effect of IL-23 on IFN-γ p...
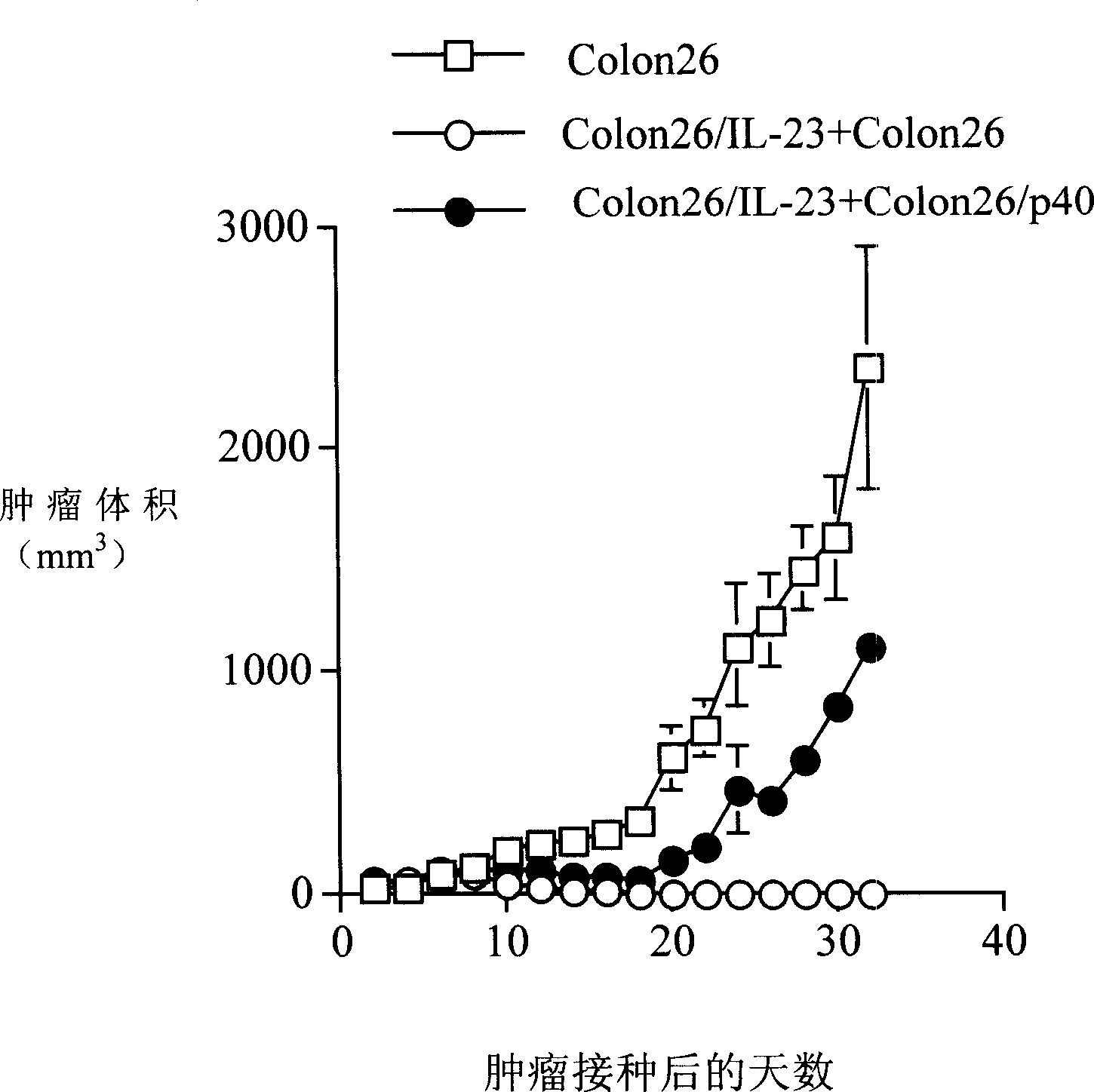
Embodiment 3
[0050] Will IL-23 or p40 gene transfer into cells
[0051] Mouse p19 and p40 cDNAs were inserted into the cloning site (Eco RI / Bam HI) of the retroviral vector LXSN using IRES. The IL-23 expression vector is a gene fragment arranged in the sequence p19 / IRES / p40 inserted downstream of the 5'LTR, and only the cDNA of p40 is inserted into the expression vector of the soluble p40 molecule. Both IL-23 and p40 transcription are controlled by the 5'LTR. After the above-mentioned expression vector was introduced into the packaging cell Psi-2 (ATCC) with Lipofectin reagent (Invitrogen Company), selection culture was carried out with a culture medium supplemented with G418 (400 μg / ml, Invitrogen Company), and then the culture supernatant and multiple Polyquaternary amine (polybrene, 8 μg / ml, Aldrich Company) was further infected with PA317 cells (ATCC), and G418 (400 μg / ml, Invitrogen Company) was used for selective culture, and the PA317 cell culture supernatant contained retroviru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com