Repurposed antibiotics for non-nuclear genotoxic chemotherapy and pharmaceutical composition for Anti-cancer containing the same
A technology of compounds and antibiotics, applied in drug combinations, chemical instruments and methods, active ingredients of phosphorus compounds, etc., to achieve the effect of preventing cancer recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0057] Synthesis Example 1: Synthesis of Ester Mt-CFX
[0058] Ester Mt-CFX was synthesized according to Scheme 1:
[0059] [Option One]
[0060]
[0061] Compounds 1 and 2 shown in Scheme 1 were synthesized according to methods known in the art.
[0062] (1) Synthesis of compound 3
[0063] Compound 1 (1 g, 1.975 mmol) and Compound 2 (852 mg, 1.975 mmol) were dissolved in dimethylformamide as a solvent, and then potassium carbonate (819 mg, 5.925 mmol) was slowly added thereto. The mixture was stirred at 50°C for 12 hours. The reaction mixture was evaporated under reduced pressure to remove solvent. The residue was purified by column chromatography and dissolved in 50 mL of a mixed solution of methanol and distilled water (1 / 9). Add NaBF to the solution 4 , followed by stirring for 1 hour. The reaction mixture was extracted with dichloromethane and distilled water and dissolved in methanol. adding After 1 x 8 chloride, the resulting mixture was stirred for 6 ho...
Synthetic example 2
[0067] Synthesis Example 2: Synthesis of Amide Mt-CFX
[0068] The amide Mt-CFX was synthesized according to Scheme 2:
[0069] [Option II]
[0070]
[0071] Compounds 1 and 2 shown in Scheme 2 were synthesized according to methods known in the art.
[0072] (1) Synthesis of Compound 4
[0073] Compound 1 (1 g, mmol) was dissolved in 35 mL of 7N NH in methanol 3in solution. The solution was stirred at room temperature for 3 days. Thereafter, the reaction solution was evaporated under reduced pressure to remove the solvent. The residue was purified by column chromatography.
[0074] (2) Synthesis of compound 5
[0075] Compound 2 (1 g, 2.318 mmol), EDC hydrochloride (667 mg, 3.477 mmol) and 1-hydroxybenzotriazole hydrate (470 mg, 3.477 mmol) were dissolved in dimethylformamide as a solvent. The solution was stirred at room temperature for 30 minutes. To the reaction solution were added DMAP (425 mg, 3.477 mmol) and compound 4 (1.025 g, 2.318 mmol). The resulting ...
experiment example
[0080] 1-1. Animal cell culture
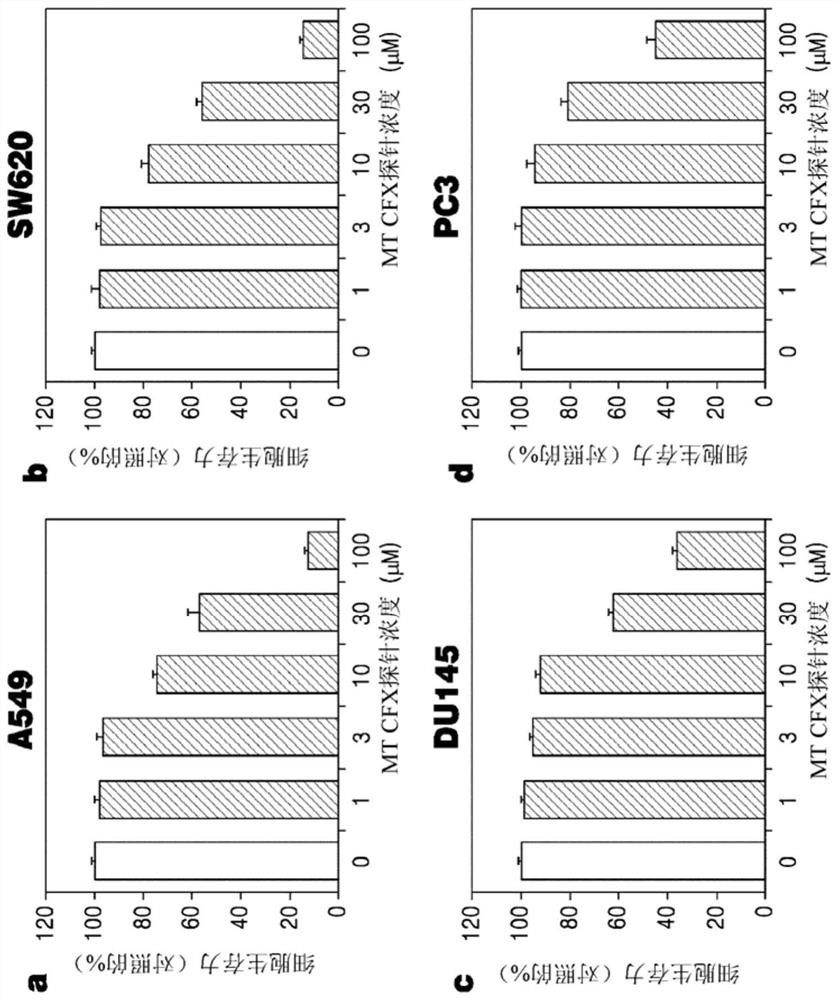
[0081] Human metastatic breast cancer cell line MDA-MB-231 (human breast cancer cells), lung cancer cell line A549 (human lung cancer cells), human colon cancer cell line SW620 (human colon cancer cells), human prostate cancer cell line DU145 and PC3 (human prostate cancer cells) were cultured in RPMI1640 medium and modified Eagle's medium (modified Eagle's media, MEM). All media were supplemented with 10% inactivated fetal bovine serum (FBS) and 1% penicillin-streptomycin. All cell lines were cultured at 37°C and 5% carbon dioxide.
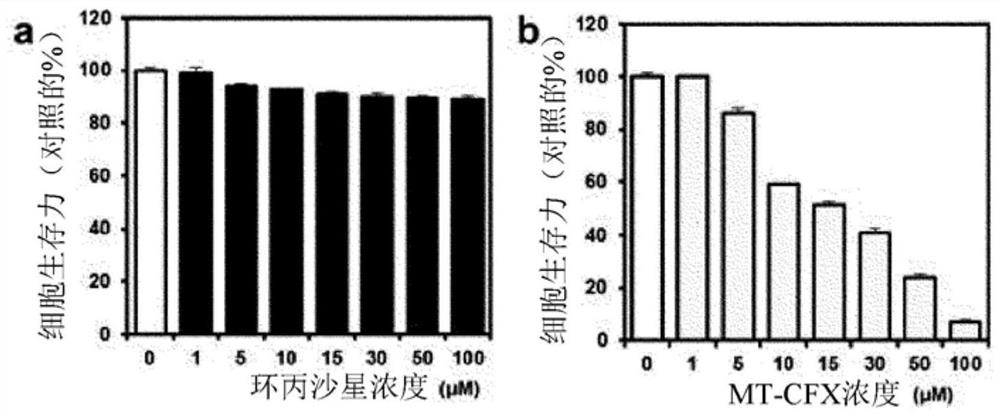
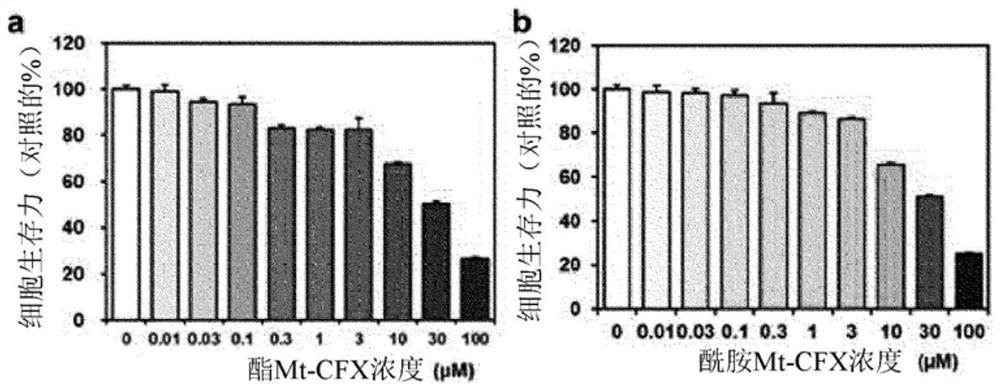
[0082] 1-2. Cell viability analysis
[0083] MDA-MB-231, A549, SW620, DU145 and PC3 cells were seeded into 96-well plates and cultured overnight for stabilization. After the stabilized cells were treated with different concentrations of ester MT-CFX, amide MT-CFX and ciprofloxacin from 0 to 100 μM for 48 hours, the amount of LDH in living cells was measured to determine cell viability. Data are shown as m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com