COMPOSITIONS AND METHODS REGARDING ENGINEERED AND NON- ENGINEERED gamma delta-T CELLS FOR TREATMENT OF HEMATOLOGICAL TUMORS
A technology of binding regions and binding domains, applied in chemical instruments and methods, blood/immune system cells, biochemical equipment and methods, etc., can solve uncertain problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
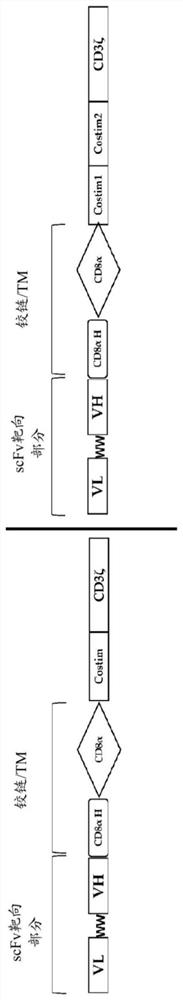
[0183] In the presence of IL-2 (100 U / mL), 1×10 6 Human PBMC / mL were activated for 5 days in modified cell culture medium on pre-coated anti-Vδ1 antibody D1-08 or D1-35 (see WO 2017 / 197347). On the 5th day, in the presence of fibronectin (retronectin), with coding chimeric antigen receptor (2B7-5.1, SEQ ID NO:11; 3B9-5.1, SEQ ID NO:9; 3H7-5.1, SEQ ID NO:9; 3H7-5.1, SEQ ID NO:9; ID NO: 10; 9C11-5.1, SEQ ID NO: 12) gamma-retroviral construct to transduce cell cultures. On day 6, cells were returned to modified cell culture medium and further expanded with feed and IL-2 replacement as needed. On day 17, 18 or 19, cells were harvested and used A kit (Miltenyi Biotec) was used to deplete remaining αβ T cells. Purity and transduction efficiency of γδ cell populations were assessed by FACS. In parallel, non-transduced cell cultures were expanded in the same manner without addition of retroviral supernatant. Such as image 3 As shown, untransduced expanded Vδ1 cells from multip...
Embodiment 2
[0185] Vδ1 cells were activated, transduced and expanded in the same manner as above. The 3H7 CAR construct SEQ ID NO: 10 was used to demonstrate cytotoxicity against two CD20+ cell lines Daudi and Raji. Such as Figure 4 As shown, the introduction of CAR enhanced the innate cytotoxicity of unengineered Vδ1 cells.
Embodiment 3
[0187] Vδ1 cells were activated, transduced and expanded in the same manner as above. During expansion, four different constructs (SEQ ID NO: 9, 10, 11, 12) were introduced into Vδ1 cells and tested against Raji-Luc cells. Cytotoxicity was determined by total luminescence measurement after addition of the luminescent substrate D-luciferin (Perkin Elmer) after co-incubation for 18 hours at different E / T ratios. Such as Figure 5 As shown, anti-CD20 CAR cells comprising the 4-1BB co-stimulatory endodomain described herein exhibit robust cell killing activity against Raji cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com