Degradation agents for cell cycle-dependent kinases, preparation method thereof, pharmaceutical compositions and use thereof
A technology of compounds and hydrates, which can be used in drug combinations, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
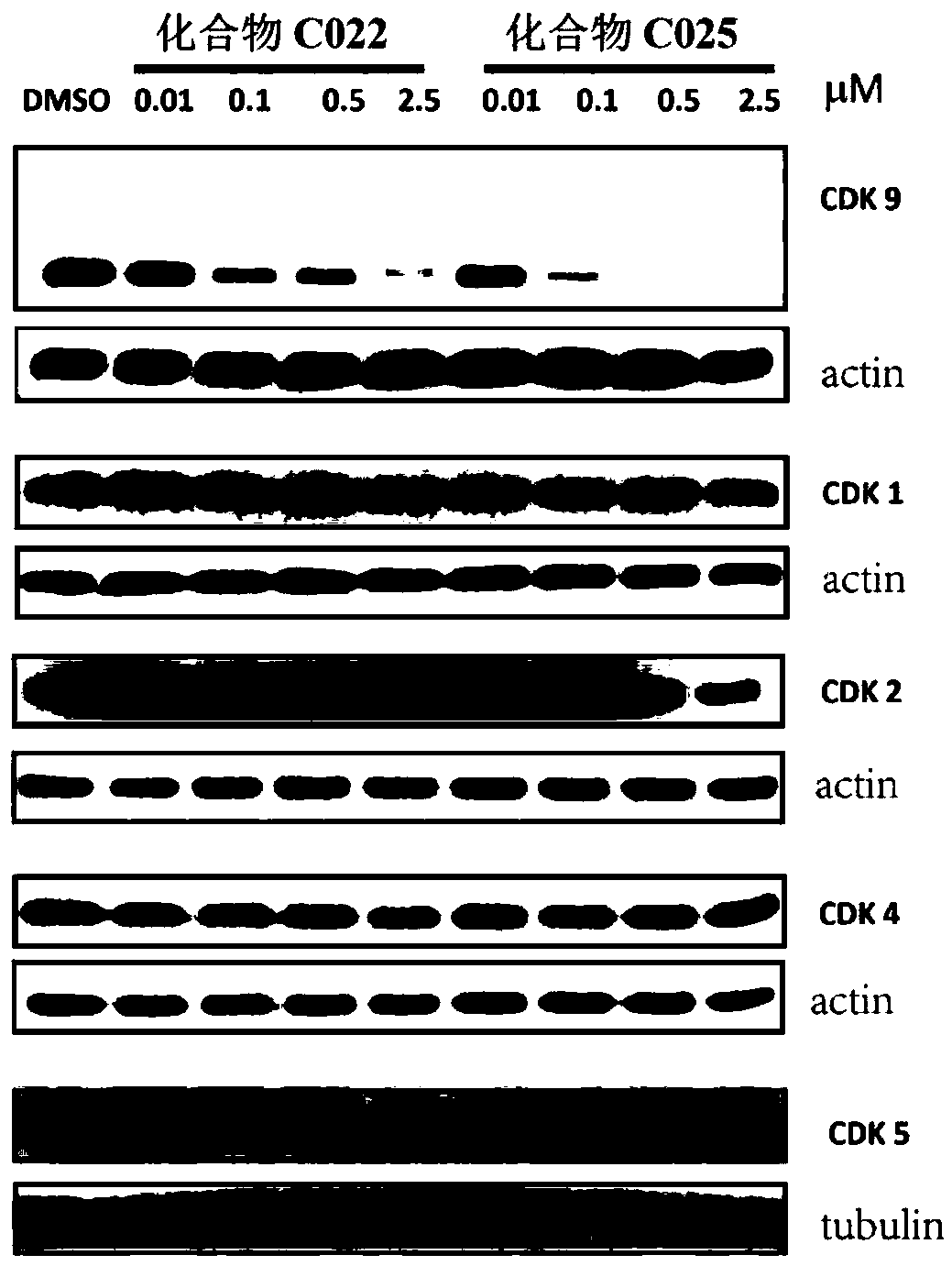
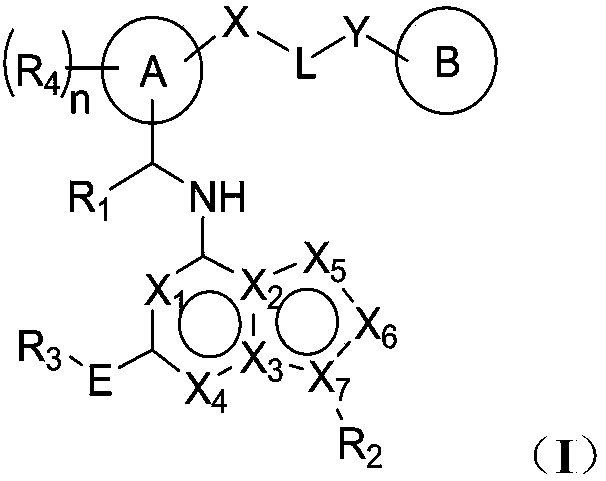
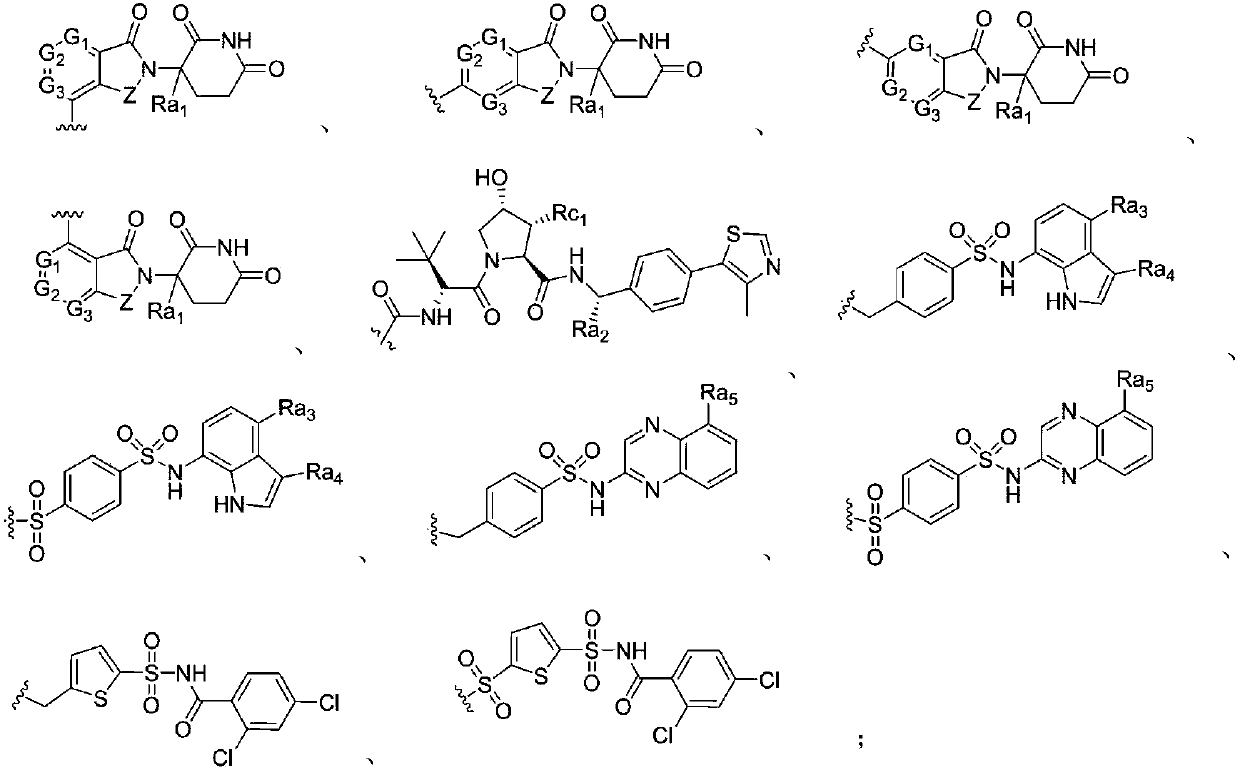
Image
Examples
Embodiment 1
[0208] [C001]N-(6-(4-(((5-((6-n-hexylamino)amino)-3-isopropylpyrazolo[1,5-a]pyrimidin-7-yl)amino) Methyl)phenyl)n-hexyl)-2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-4-yl)oxy) Preparation of acetamide
[0209]
[0210] Dissolve p-bromobenzylamine (1g, 5.37mmol) in dichloromethane, add triethylamine (1.23mL, 5.37mmol, 1 equiv), add di-tert-butyl dicarbonate dropwise at 0°C, and react at room temperature for 12 hours. Dichloromethane was added to the reaction solution, saturated NaHCO 3 Wash, anhydrous Na 2 SO 4 After drying and column chromatography, the product 1.1 (1.35 g) was obtained with a yield of 88%. 1 H NMR (400MHz, CDCl 3 ) δ 7.43 (d, J=7.5Hz, 2H), 7.15 (d, J=8.0Hz, 2H), 4.25 (d, J=5.6Hz, 2H), 1.45 (s, 9H).
[0211] 6-Chlorohexyne (1g, 8.58mmol), potassium phthalimide (2.54g, 13.72mmol, 1.6equiv) were dissolved in 10mL of DMF, reacted at 70°C for 18 hours, added water to the reaction solution, extracted with ethyl acetate , combined ethyl acetate layers...
Embodiment 2
[0223] [C012]7-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-4-yl)oxy)acetamido) -N-(4-(((8-isopropyl-2-((1-methylpiperidin-4-yl)oxy)pyrazolo[1,5-a][1,3,5] Preparation of Triazin-4-yl)amino)methyl)phenyl)heptanamide
[0224]
[0225] 4-Nitrobenzylamine hydrochloride was dissolved in (590 mg, 3.13 mmol) in 15 mL of dichloromethane, and DIPEA (1.03 mL, 6.26 mmol, 2 equiv), Fmoc-OSU (1.1 g, 3.28 mmol, 1.05 equiv) was dissolved in dichloromethane, added dropwise to the reaction solution, reacted at room temperature for 4 hours, added 100mL of dichloromethane to the reaction, washed several times with 0.5N aqueous HCl solution, washed with saturated NaCl, anhydrous NaCl 2 SO 4 After drying, filtering, removing the solvent under reduced pressure, and column chromatography, the product 2.1 (1.15 g) was obtained with a yield of 98%. 1 H NMR (400MHz, CDCl 3 )δ8.18(d, J=8.5Hz, 2H), 7.78(d, J=7.6Hz, 2H), 7.59(d, J=7.4Hz, 2H), 7.46-7.29(m, 6H), 4.54( d, J=6.4Hz, 2H), 4.45(d, ...
Embodiment 3
[0238] [C014]N-(4-(dimethylamino)-3-(((8-isopropyl-2-((1-methylpiperidin-4-yl)oxy)pyrazolo[1, 5-a][1,3,5]triazin-4-yl)amino)methyl)phenyl)-7-(2-((2-(2,6-dioxo-3-yl)- 1,3-dioxoisoindol-4-yl)oxy)acetamido)heptanamide
[0239]
[0240] 2-Fluoro-5-nitrobenzonitrile (2g, 12.04mmol), dimethylamine hydrochloride (1.96g, 24.08mmol, 2equiv), potassium carbonate (5g, 36.12mmol, 3equiv) were dissolved in DMF, and reacted at room temperature for 12 hours, Add 150mL ethyl acetate to the reaction, wash with water several times, wash with saturated NaCl, anhydrous NaCl 2 SO 4 After drying, filtering, and removing the solvent under reduced pressure, the product 3.1 (2.24 g) was obtained in a yield of 97%. 1 HNMR (400MHz, CDCl 3 ) 8.38 (d, J=2.8Hz, 1H), 8.15 (dd, J=9.6, 2.8Hz, 1H), 6.78 (d, J=9.6Hz, 1H), 3.32 (s, 6H).
[0241] Compound 3.1 (2.24 g, 11.72 mmol) was dissolved in EtOH (20 mL) / H 2 O (10mL) / THF (20mL), add iron powder (3.27g, 58.58mmol, 5equiv) and NH 4 Cl (3.13g, 58.58mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com