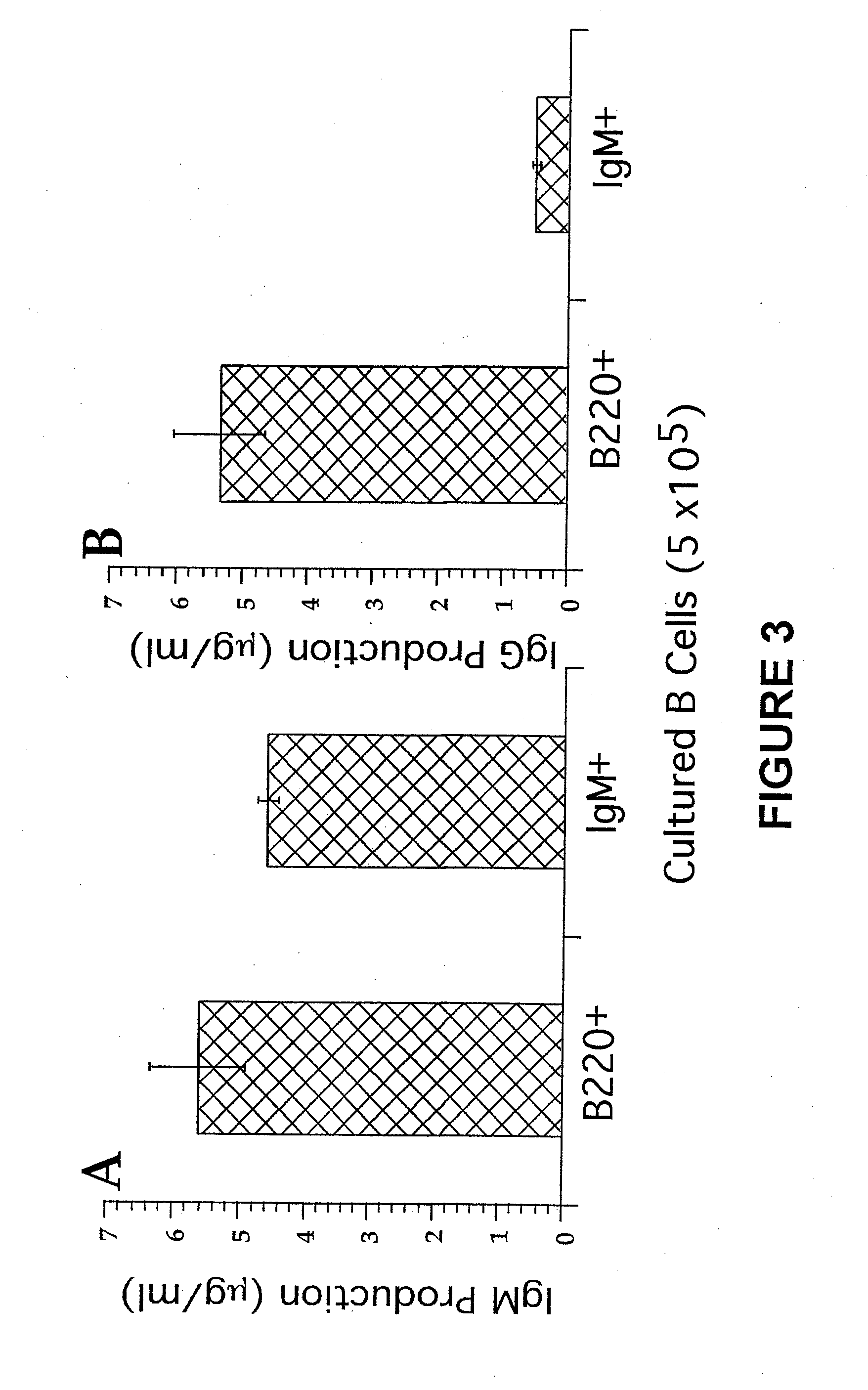
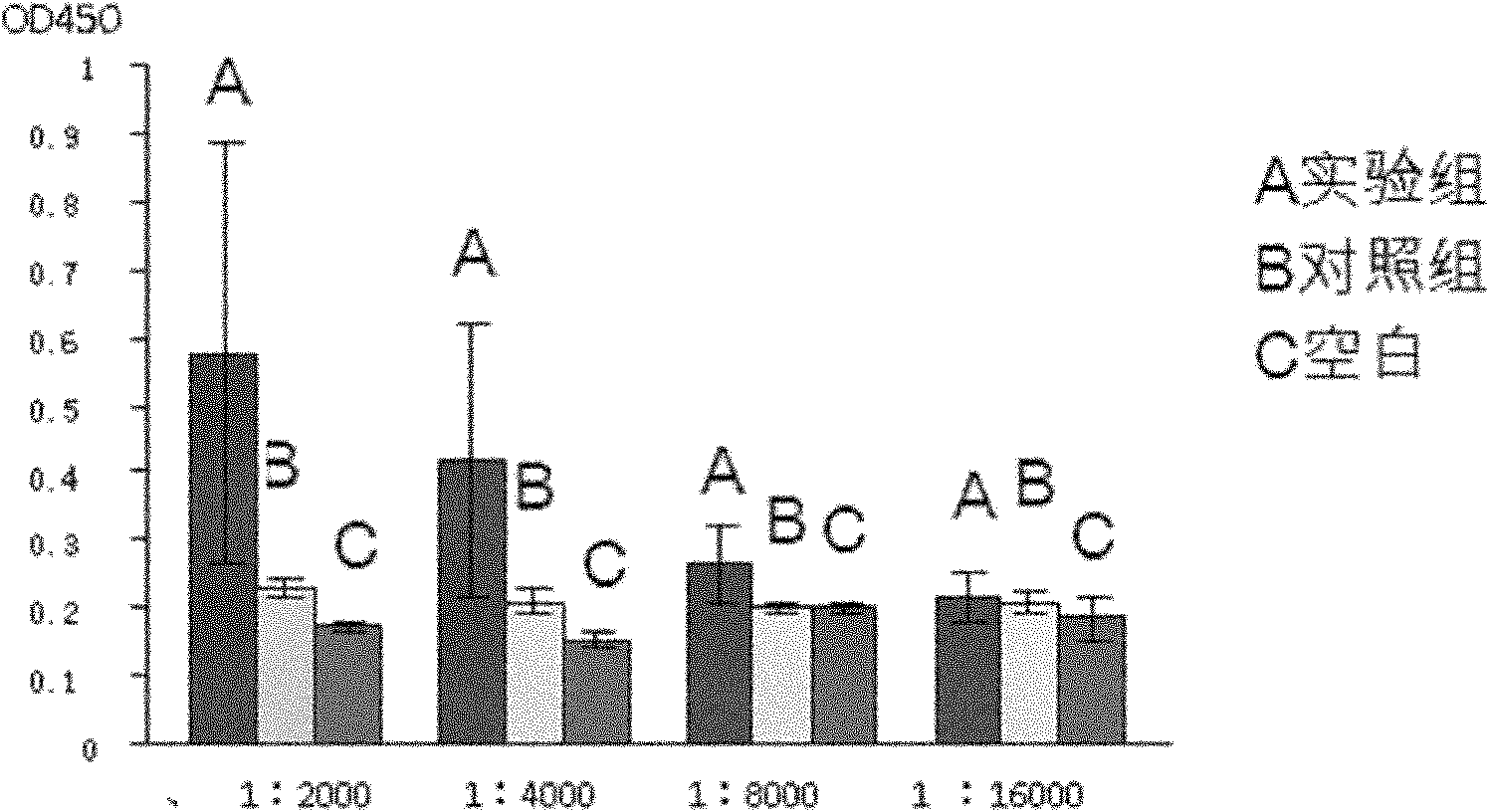
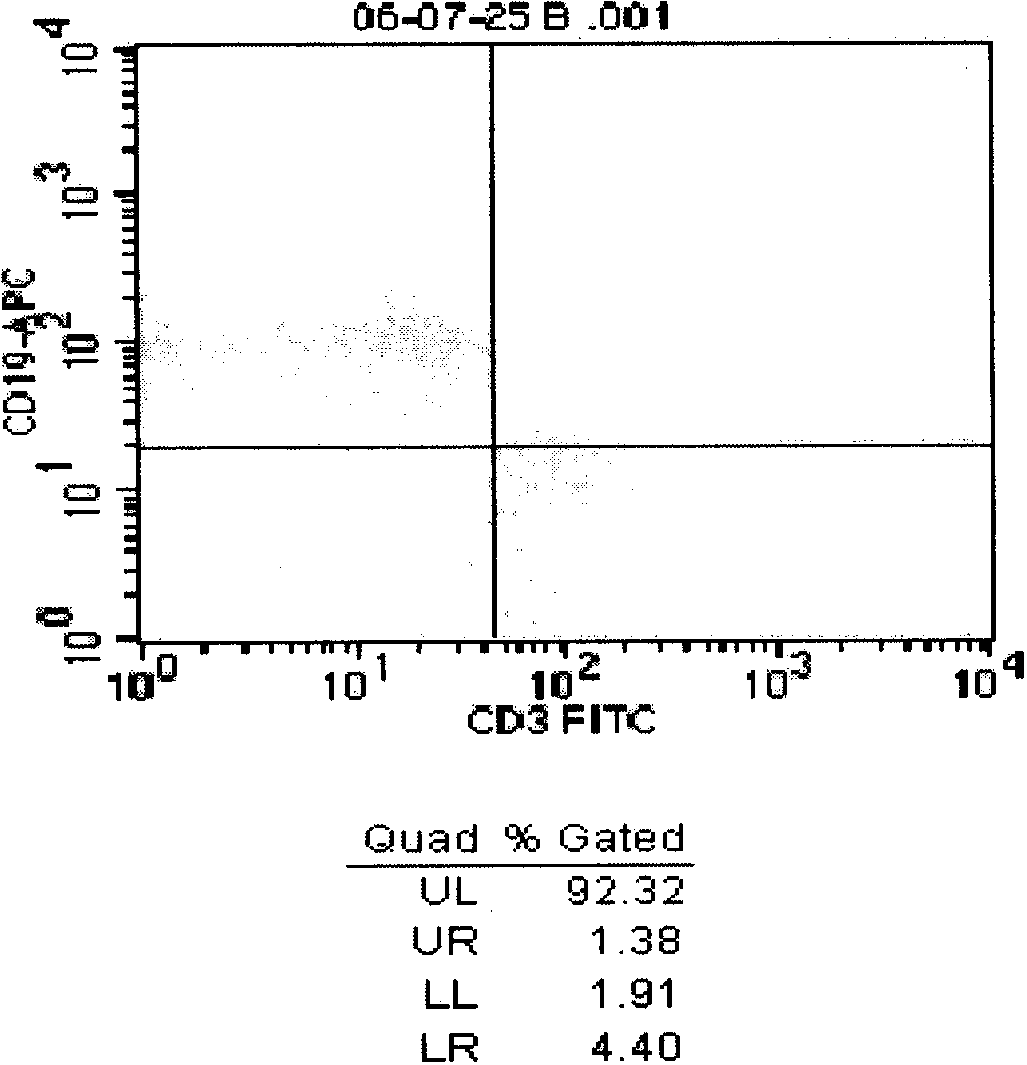
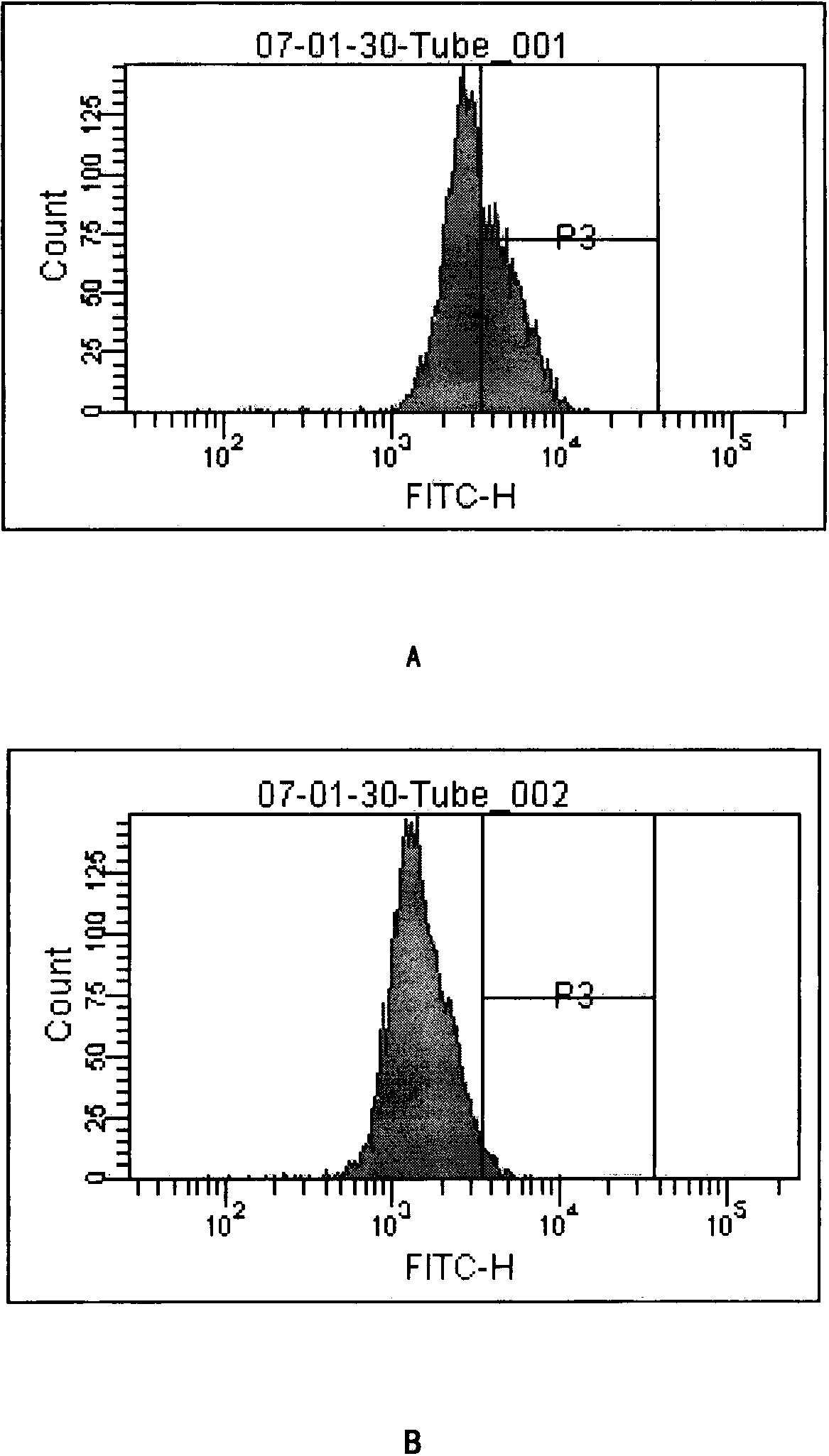
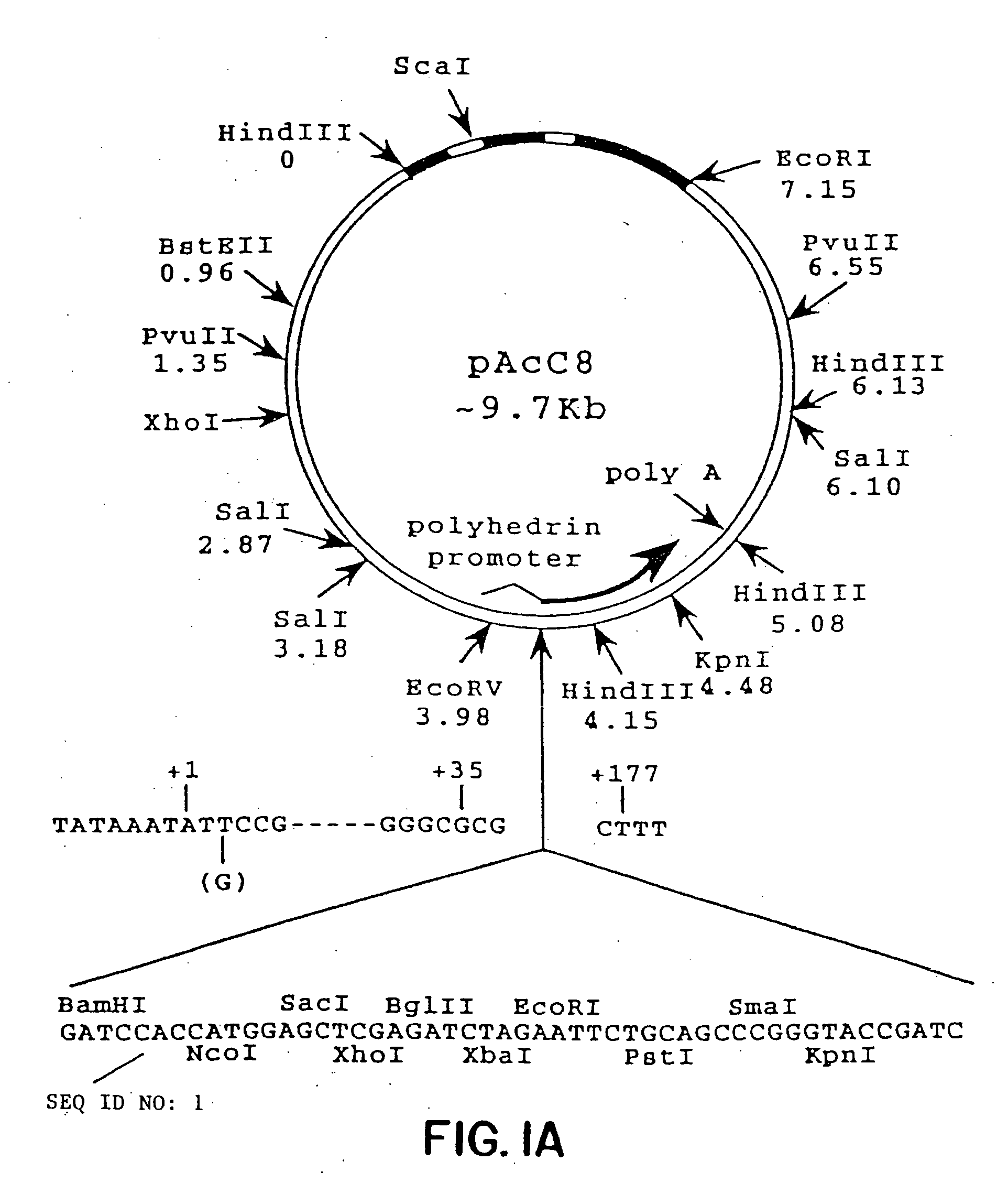
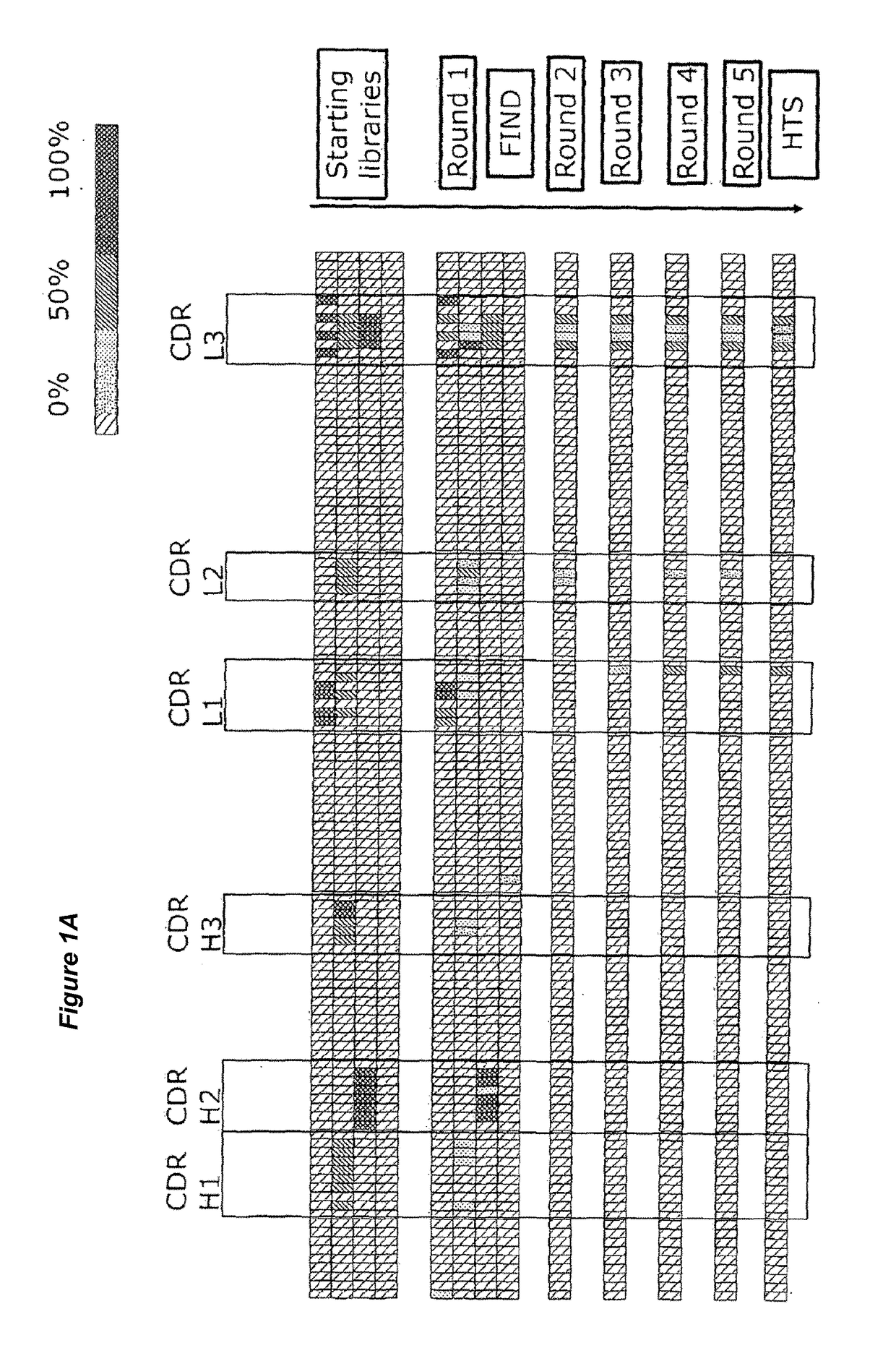
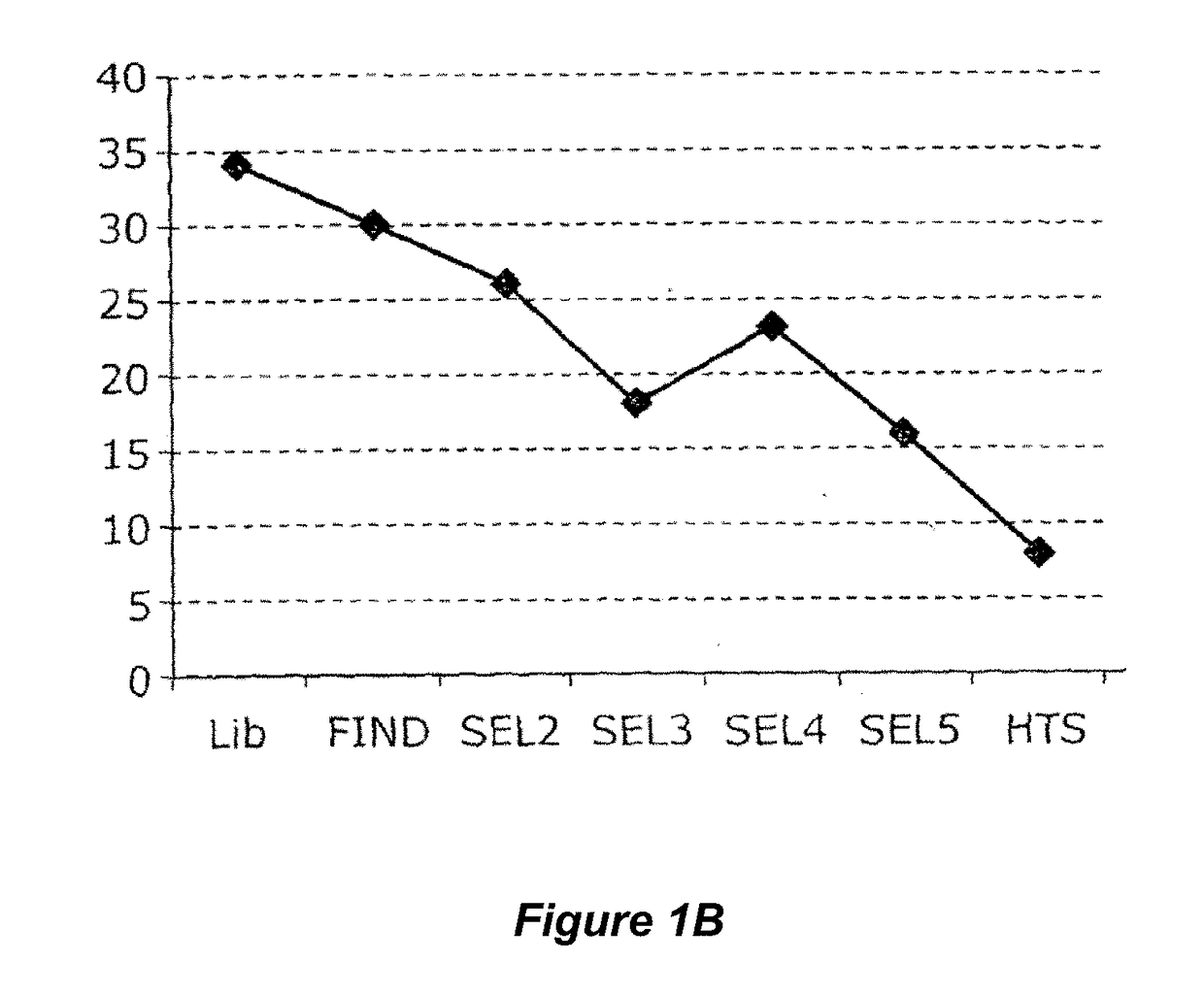
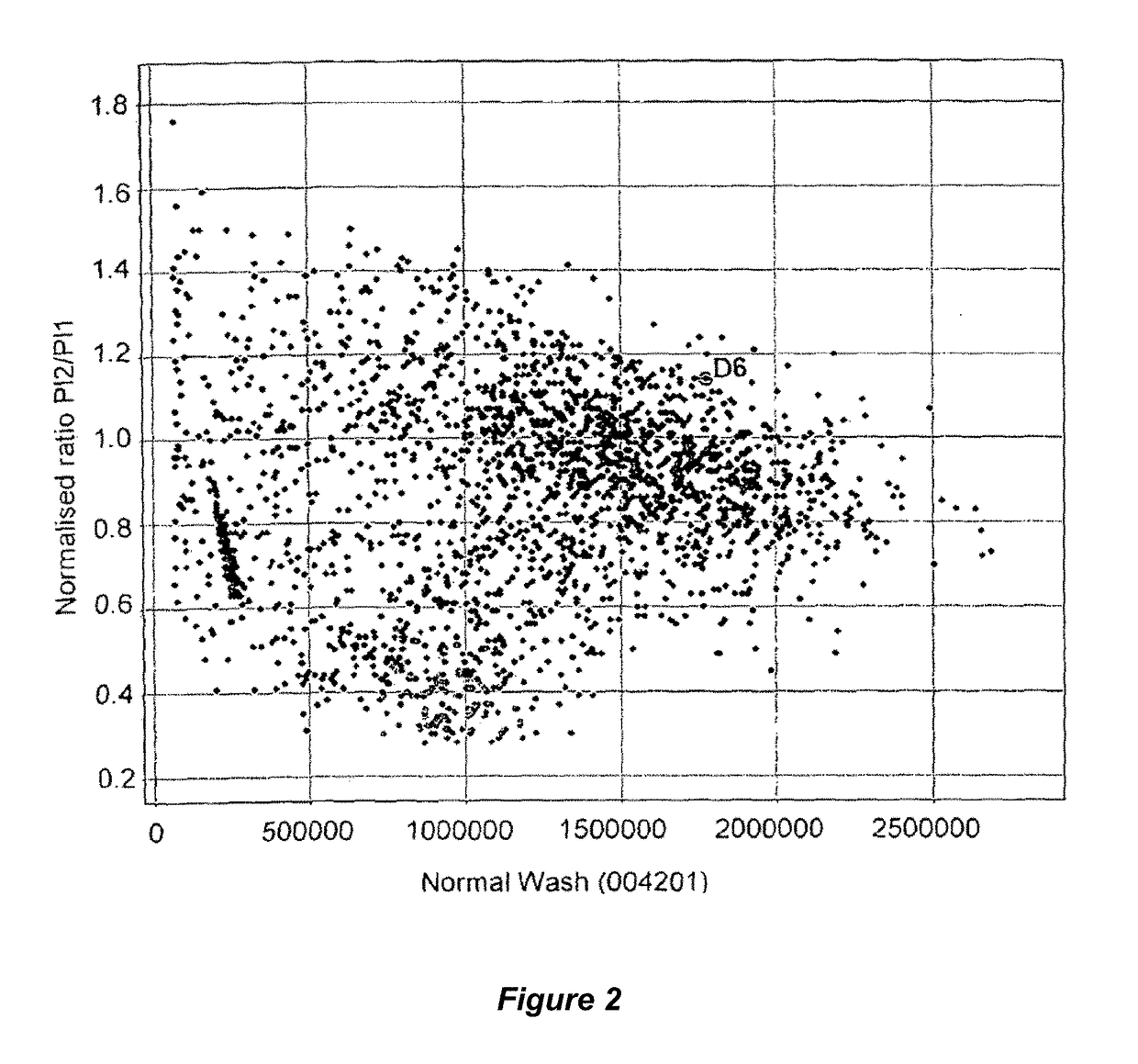
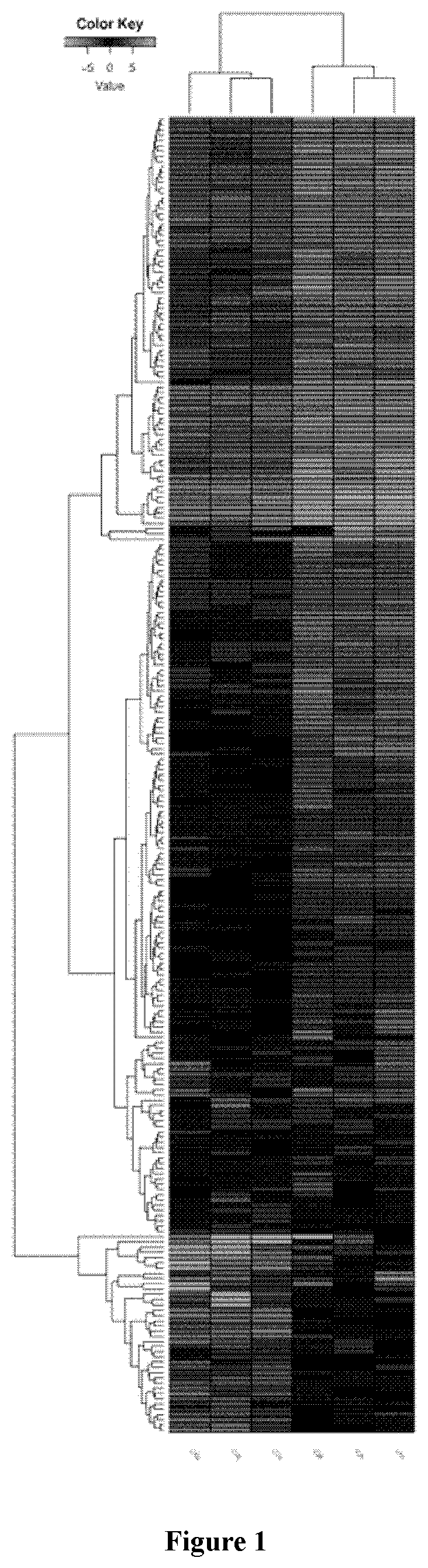
Patents
Literature
31 results about "B-cell activation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein involved in the activation and proliferation of B-cells. B-cells are activated by the binding of antigen to receptors on its cell surface which causes the cell to divide and proliferate.
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20020012665A1Avoiding and decreasing and resistanceOrganic active ingredientsIn-vivo radioactive preparationsFactor iiBiological activation
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN(R).
Owner:BIOGEN INC
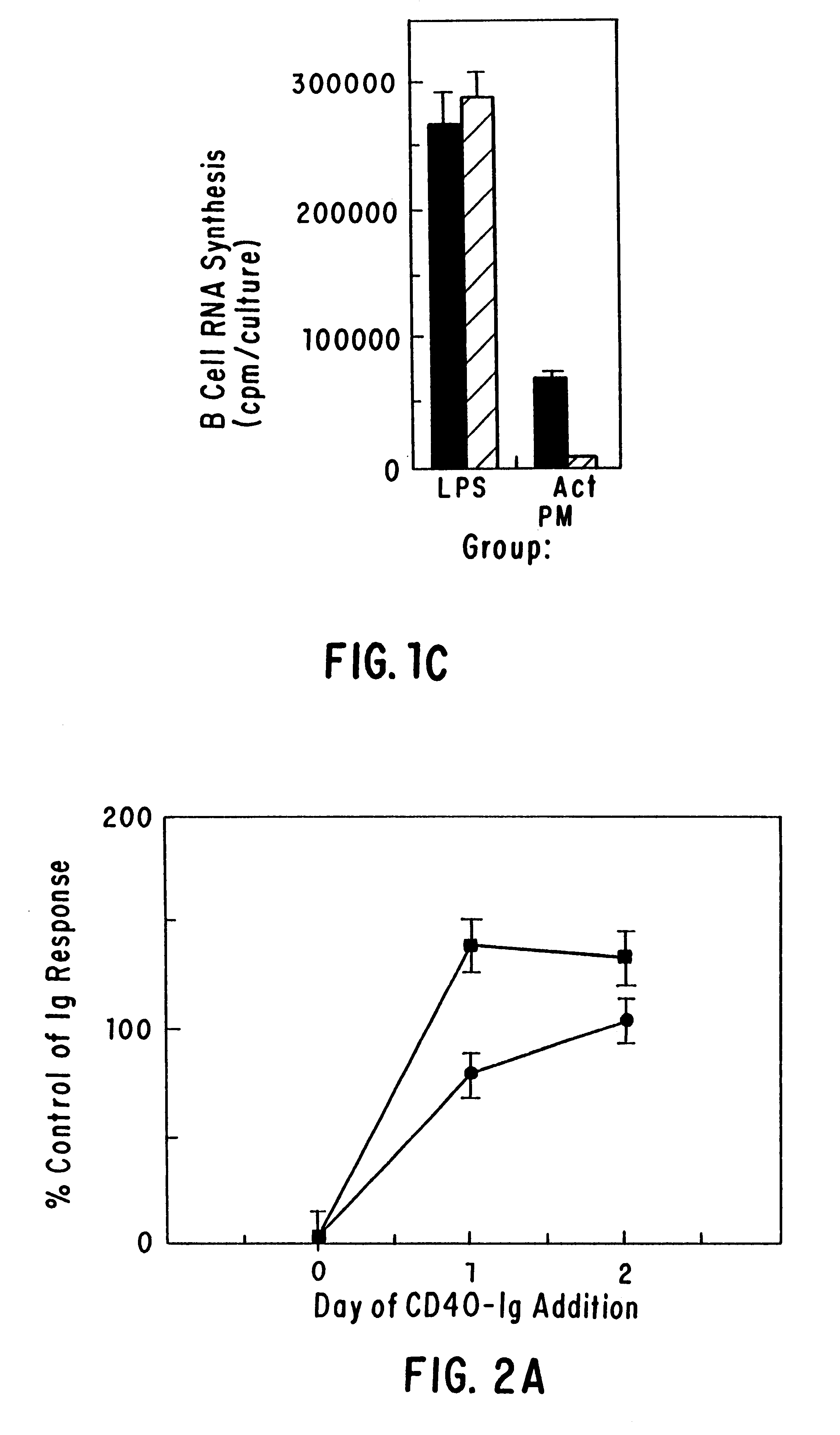
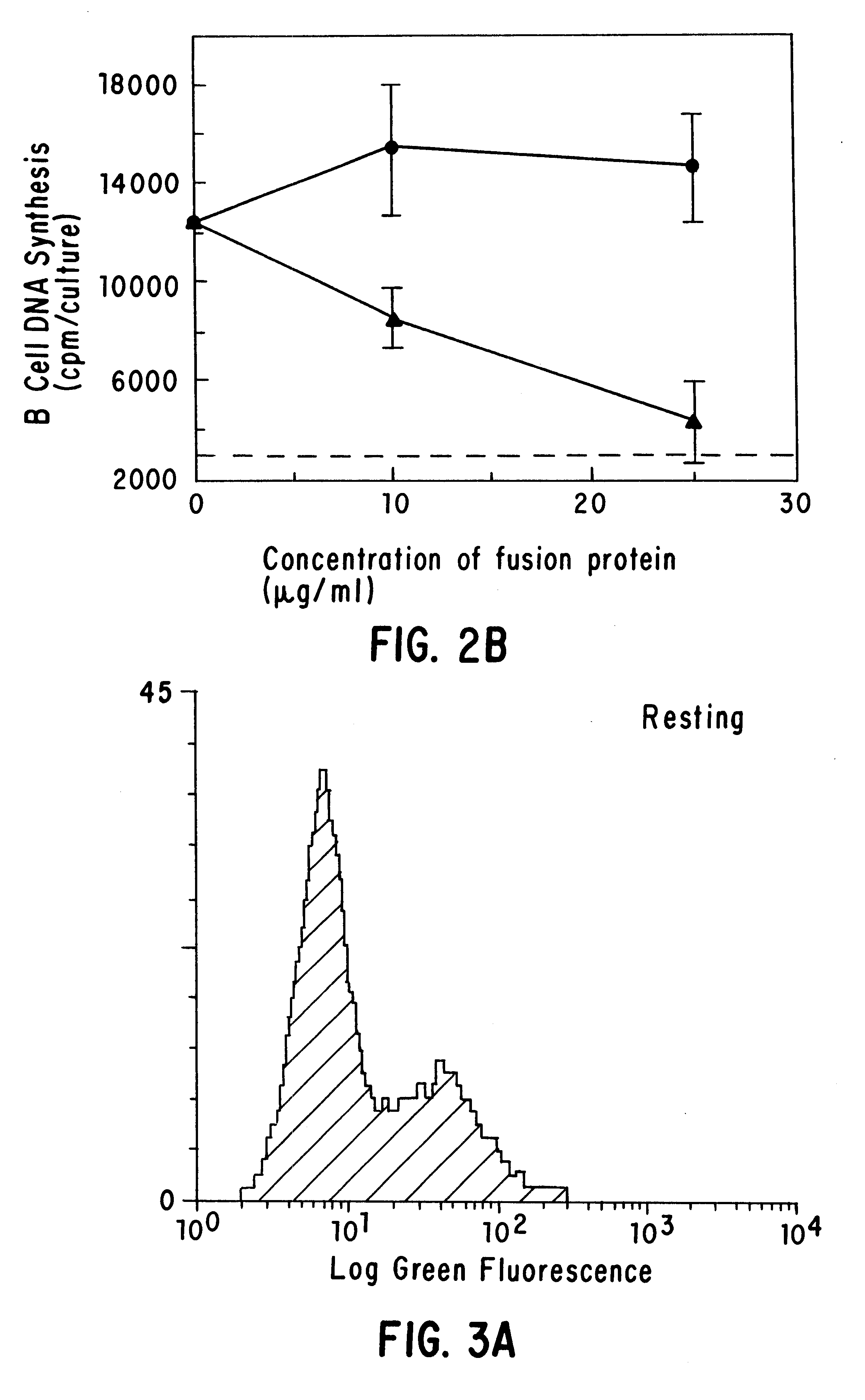
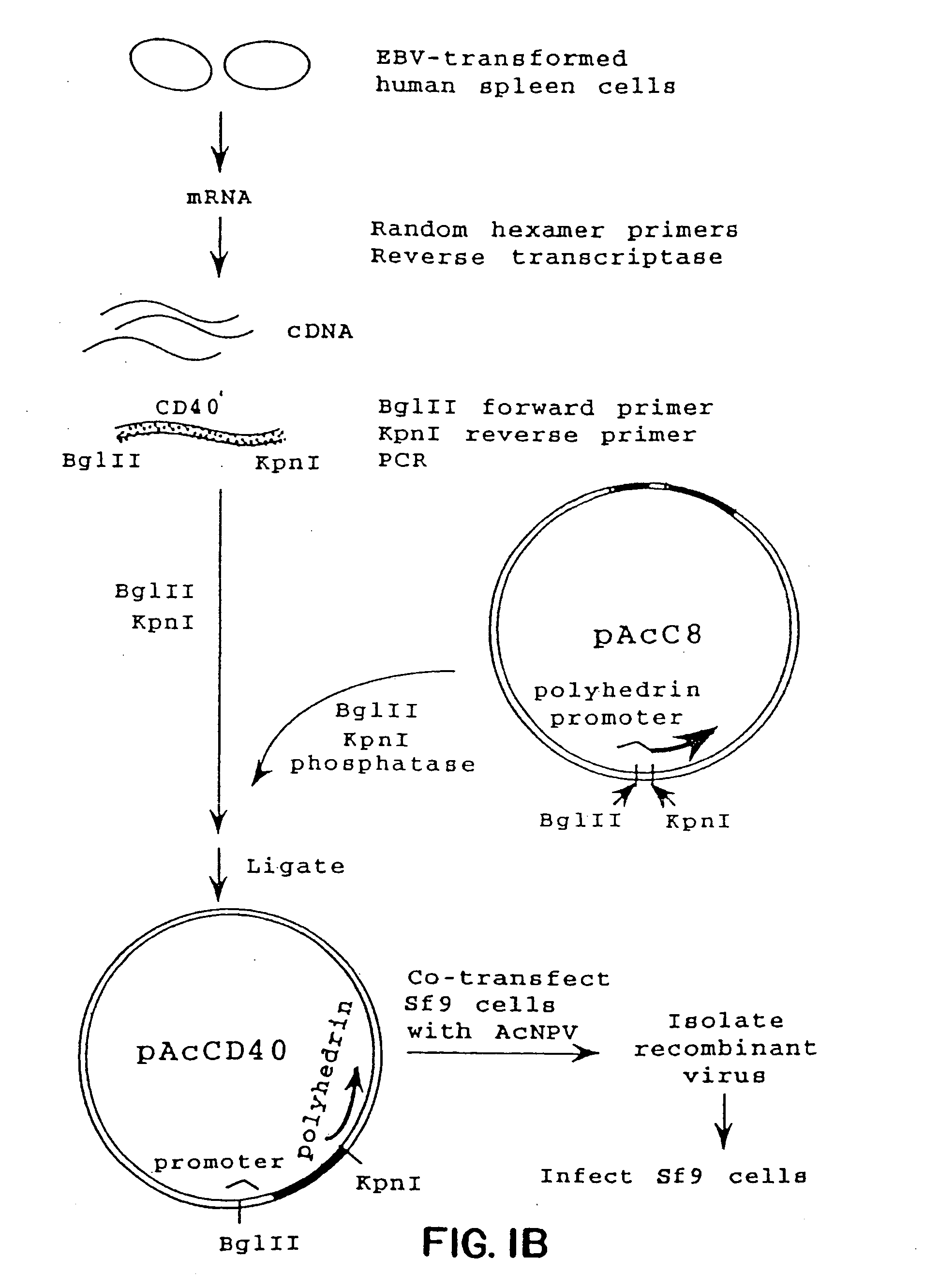
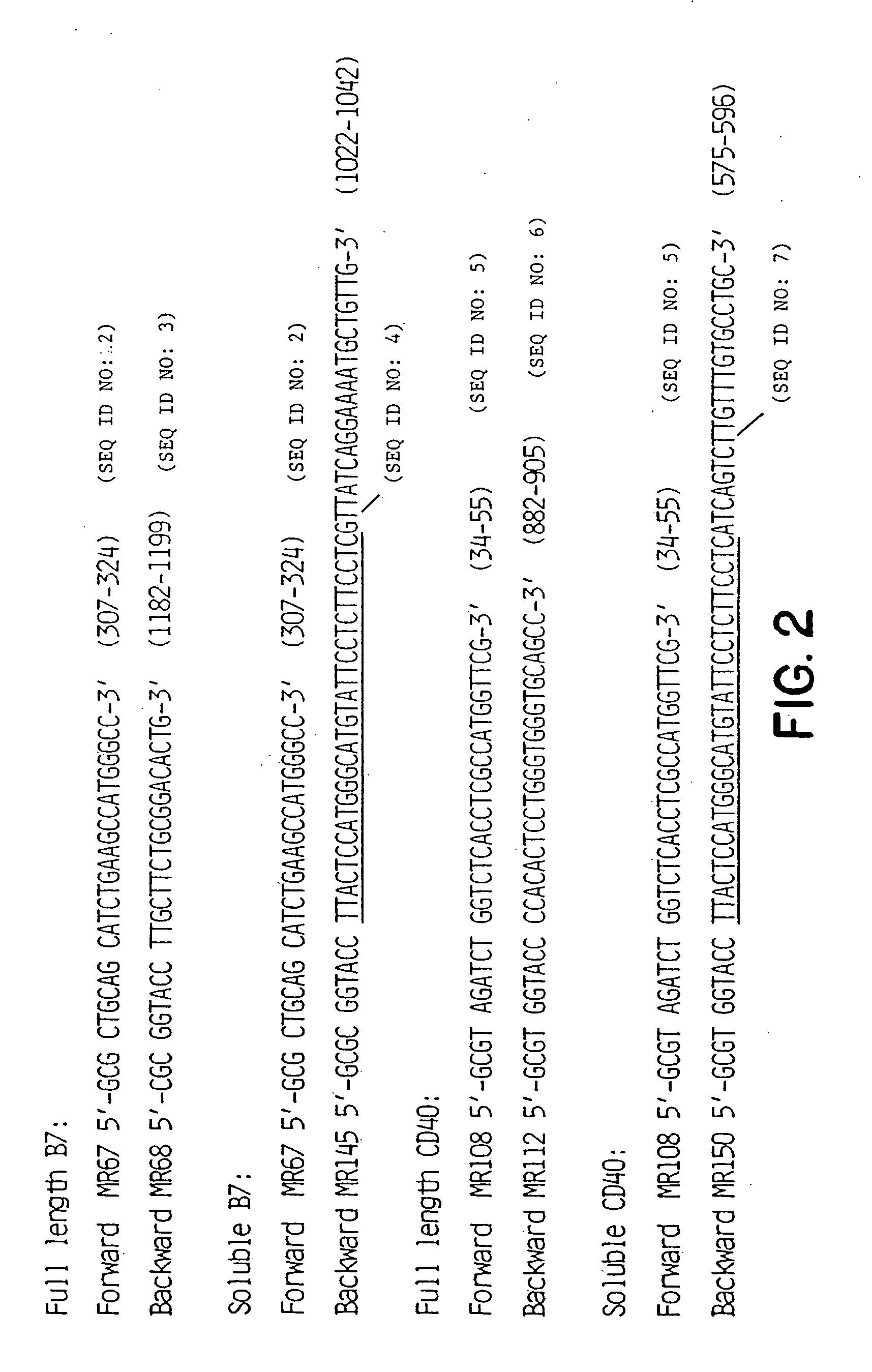
Inhibiting B cell activation with soluble CD40 or fusion proteins thereof
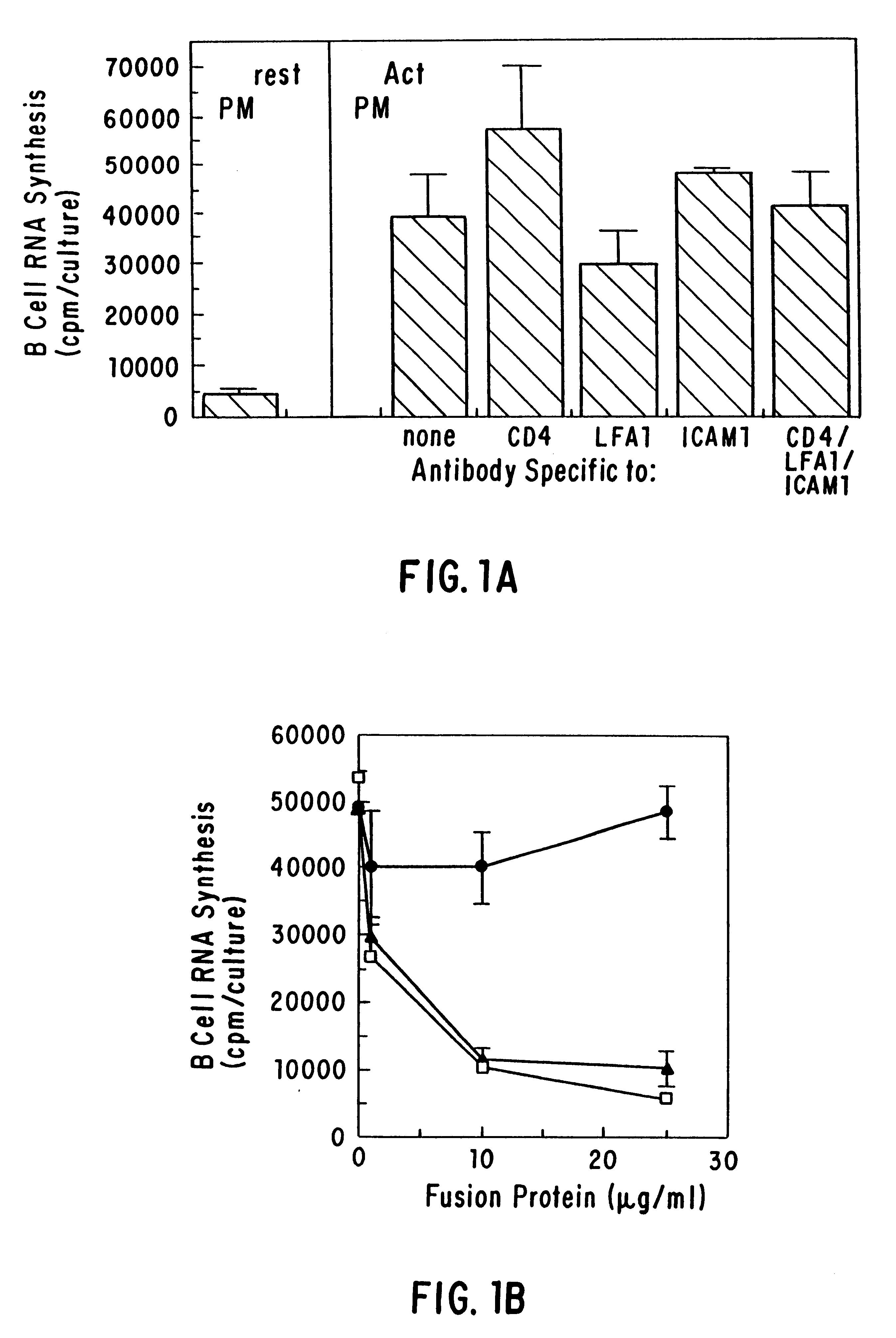
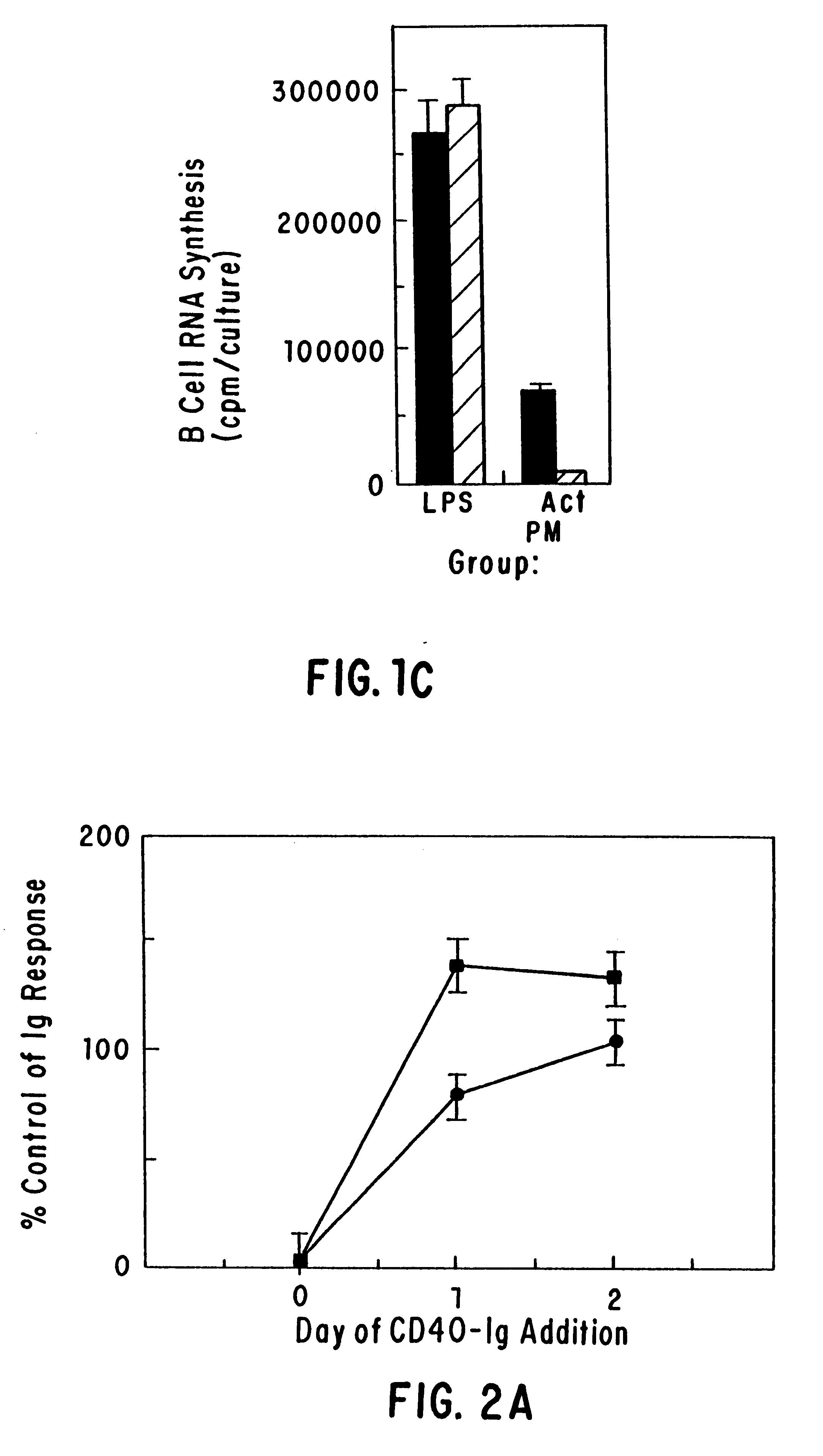
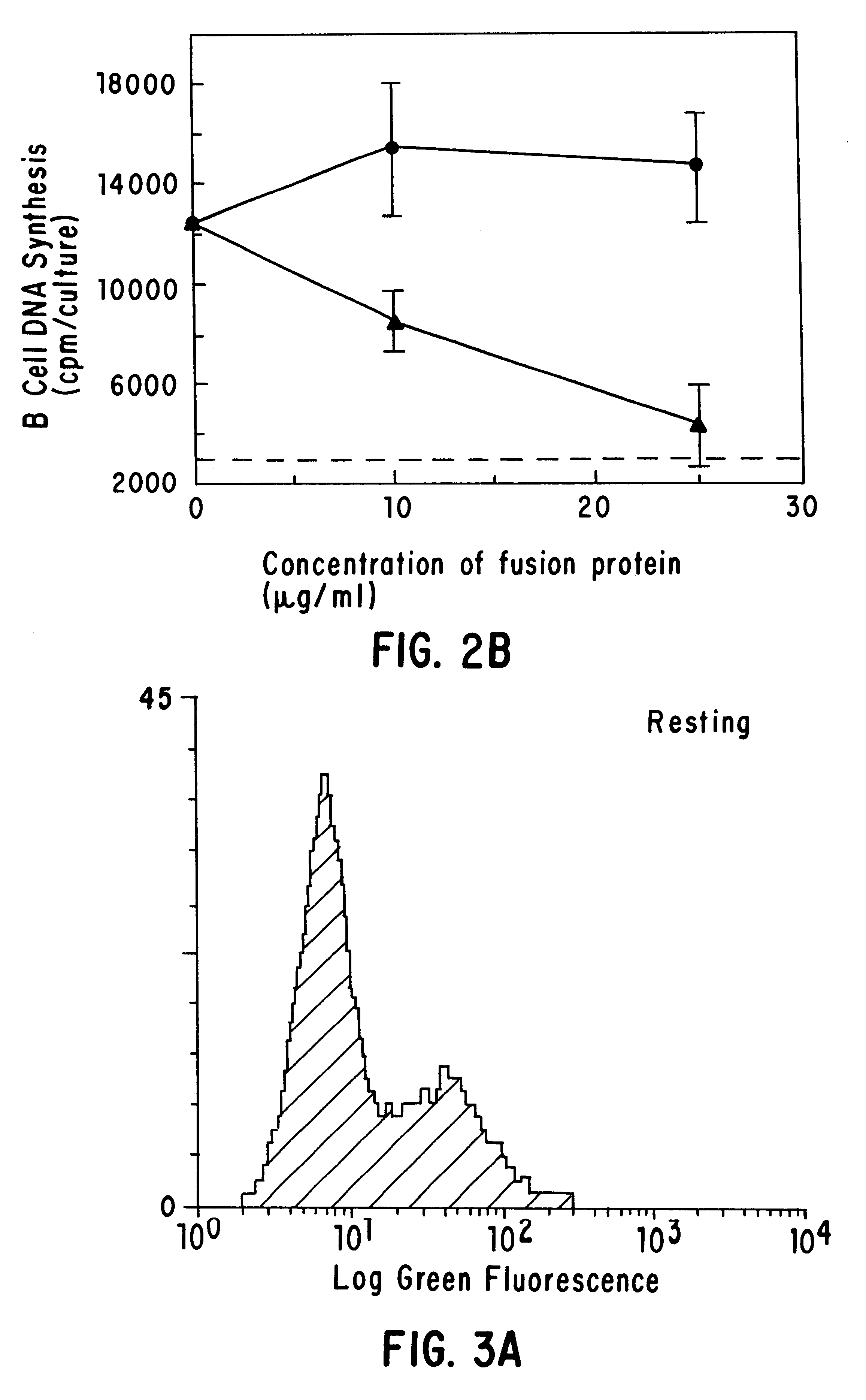
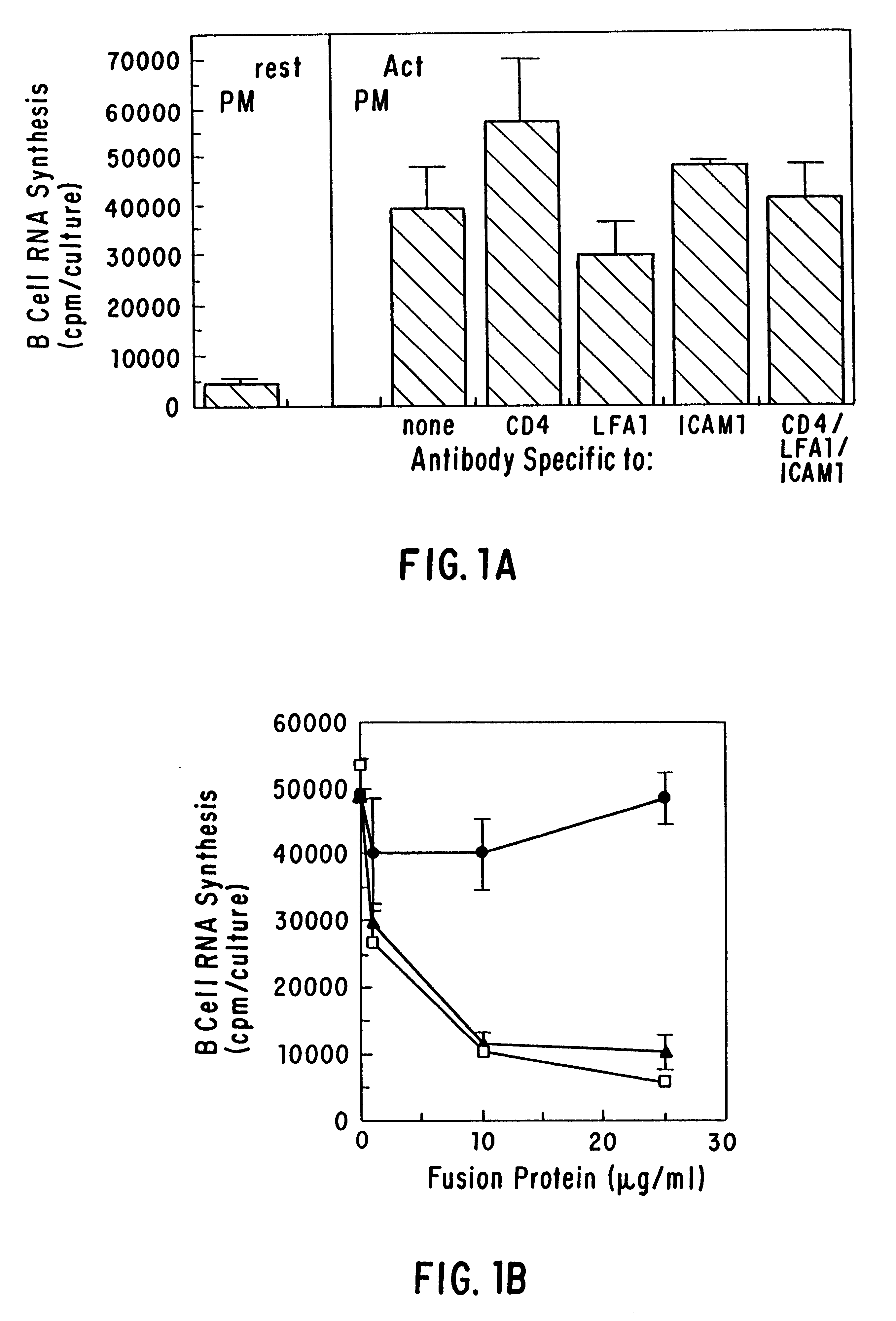
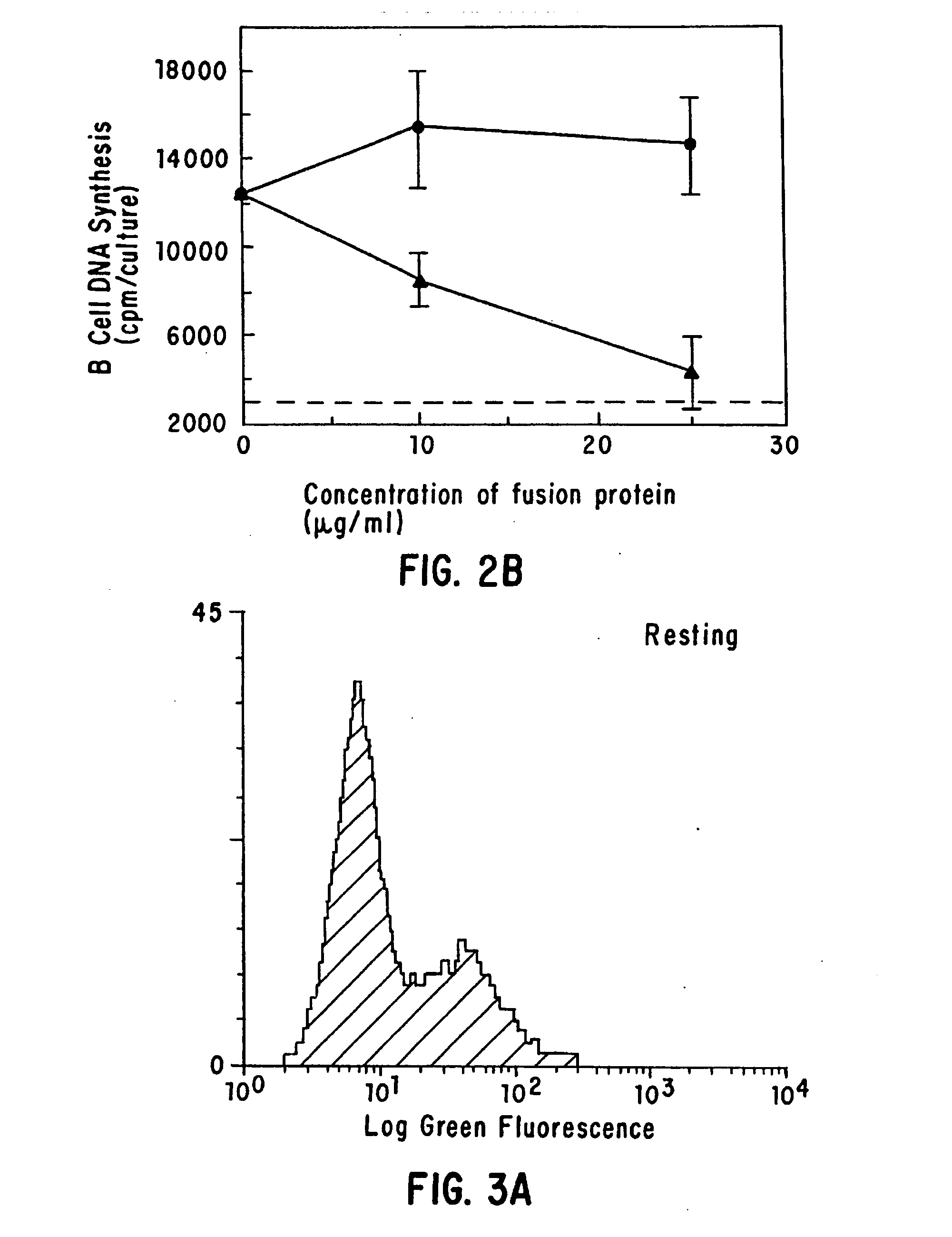
The present invention relates to a counter-receptor, termed CD40CR, for the CD40 B-cell antigen, and to soluble ligands for this receptor, including fusion molecules comprising at least a portion of CD40 protein. It is based, at least in part, on the discovery that a soluble CD40 / immunoglobulin fusion protein or antibody specific for gp39 on T cells was able to inhibit helper T-cell mediated B-cell activation by binding to a novel 39 kD protein receptor on helper T-cell membranes. The present invention provides for a substantially purified CD40CR receptor; for soluble ligands of CD40CR, including antibodies as well as fusion molecules comprising at least a portion of CD40 protein; and for methods of controlling B-cell activation which may be especially useful in the treatment of allergy or autoimmune disease, including graft-versus-host disease and rheumatoid arthritis.
Owner:BRISTOL MYERS SQUIBB CO +1
CD40 receptor ligands
InactiveUS6472510B1Analysis impossiblePeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseCell membrane
The present invention relates to a counter-receptor, termed CD40CR, for the CD40 B-cell antigen, and to soluble ligands for this receptor, including fusion molecules comprising at least a portion of CD40 protein. It is based, at least in part, on the discovery that a soluble CD40 / immunoglobulin fusion protein was able to inhibit helper T-cell mediated B-cell activation by binding to a novel 39 kD protein receptor on helper T-cell membranes. The present invention provides for a substantially purified CD40CR receptor; for soluble ligands of CD40CR, including antibodies as well as fusion molecules comprising at least a portion of CD40 protein; and for methods of controlling B-cell activation which may be especially useful in the treatment of allergy or autoimmune disease.
Owner:BRISTOL MYERS SQUIBB CO
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20050180975A1Avoiding and decreasing and resistanceIncrease ratingsBiocidePeptide/protein ingredientsBiological activationHematologic malignancy
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN®.
Owner:BIOGEN INC
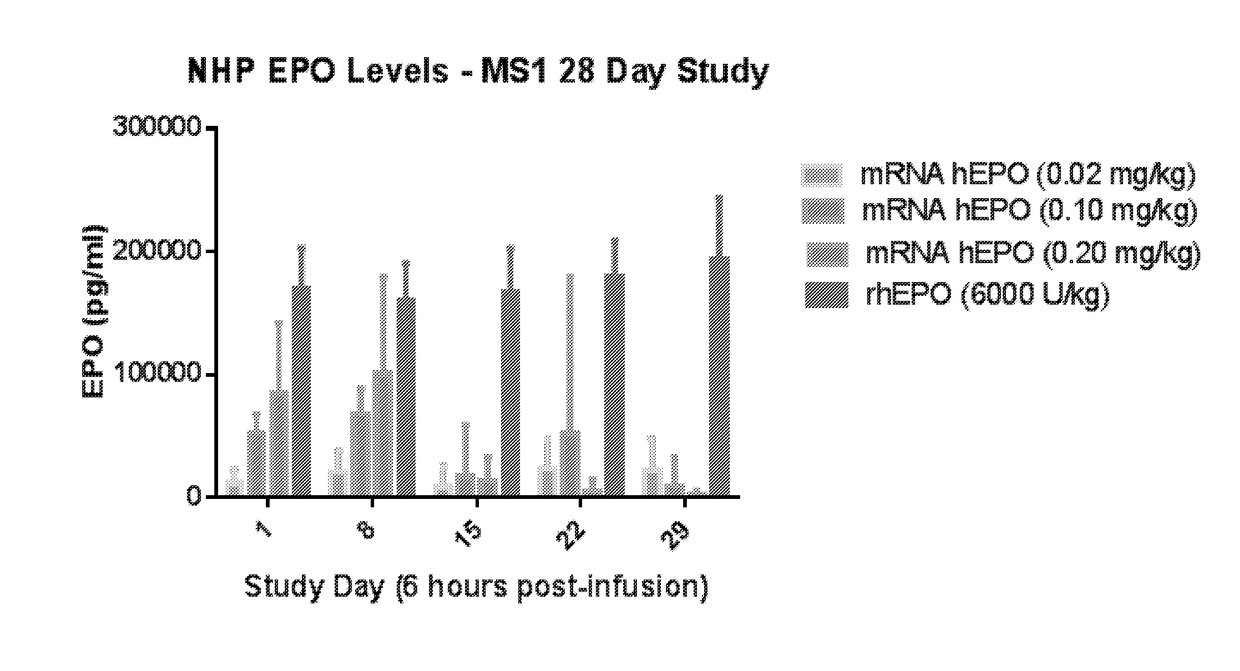
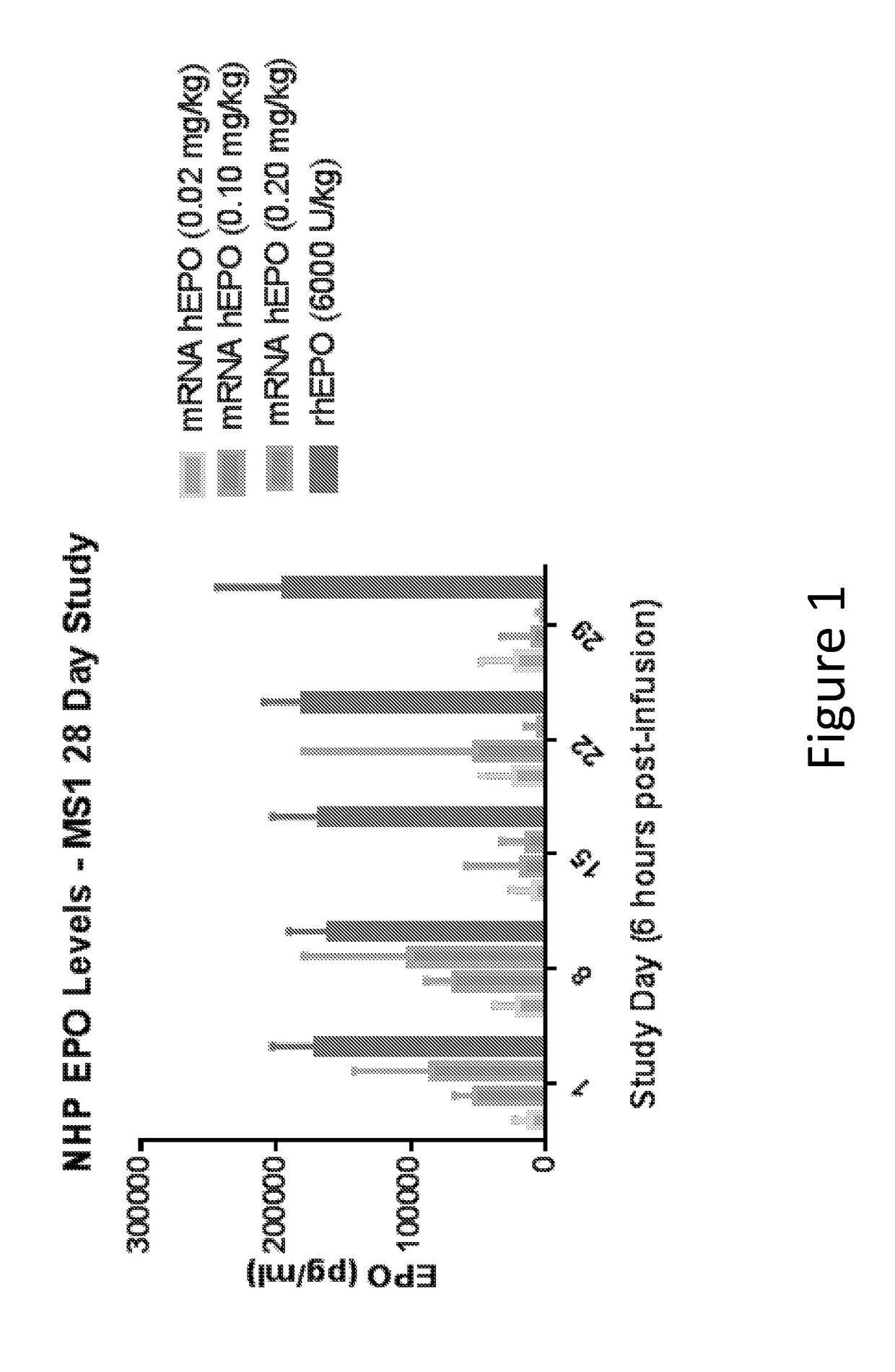
Methods for therapeutic administration of messenger ribonucleic acid drugs
ActiveUS20180256628A1Reducing and inhibiting accelerated blood clearanceBlood clearance is reduced and inhibitedOrganic active ingredientsNucleic acid vectorAntiendomysial antibodiesBinding site
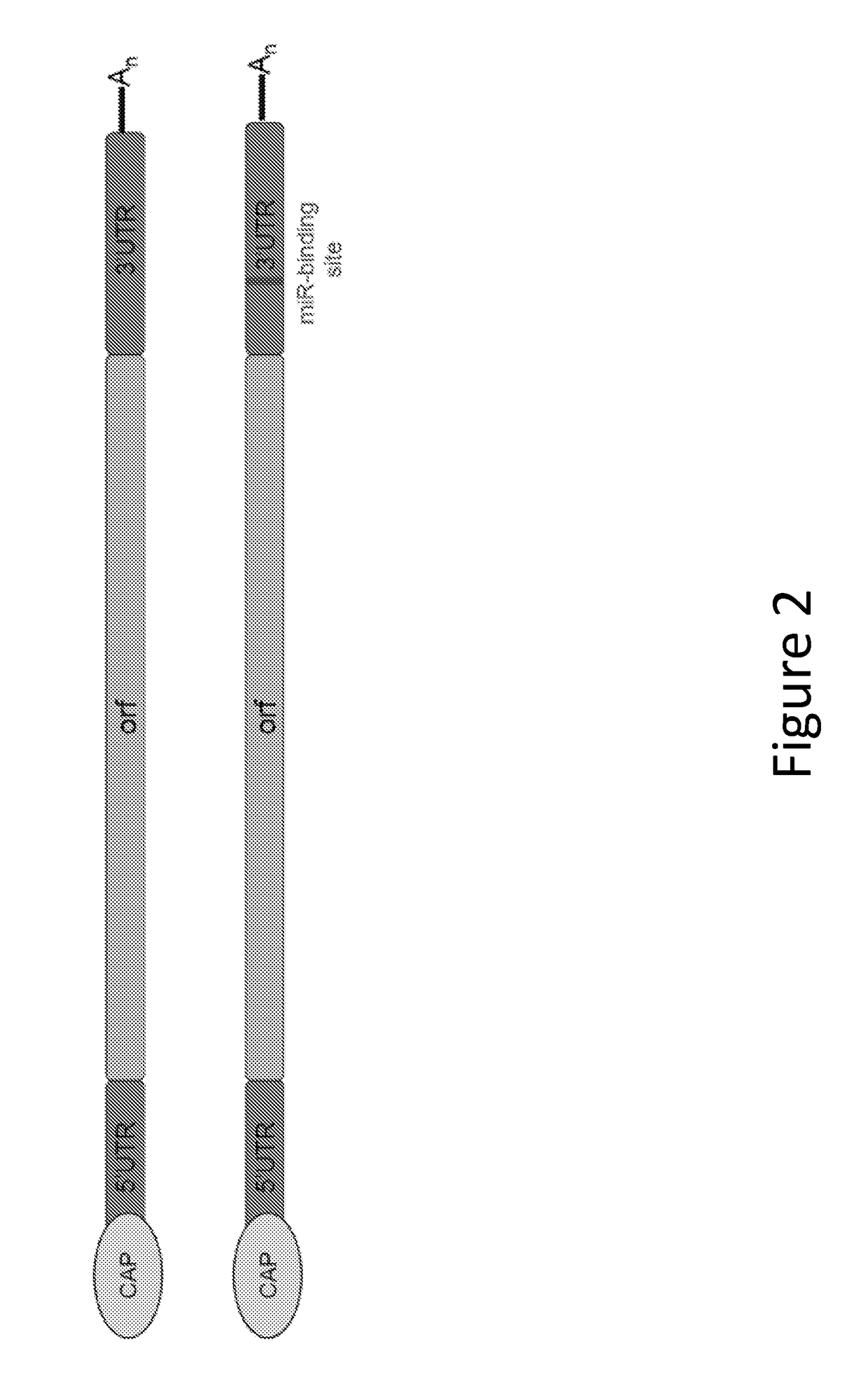
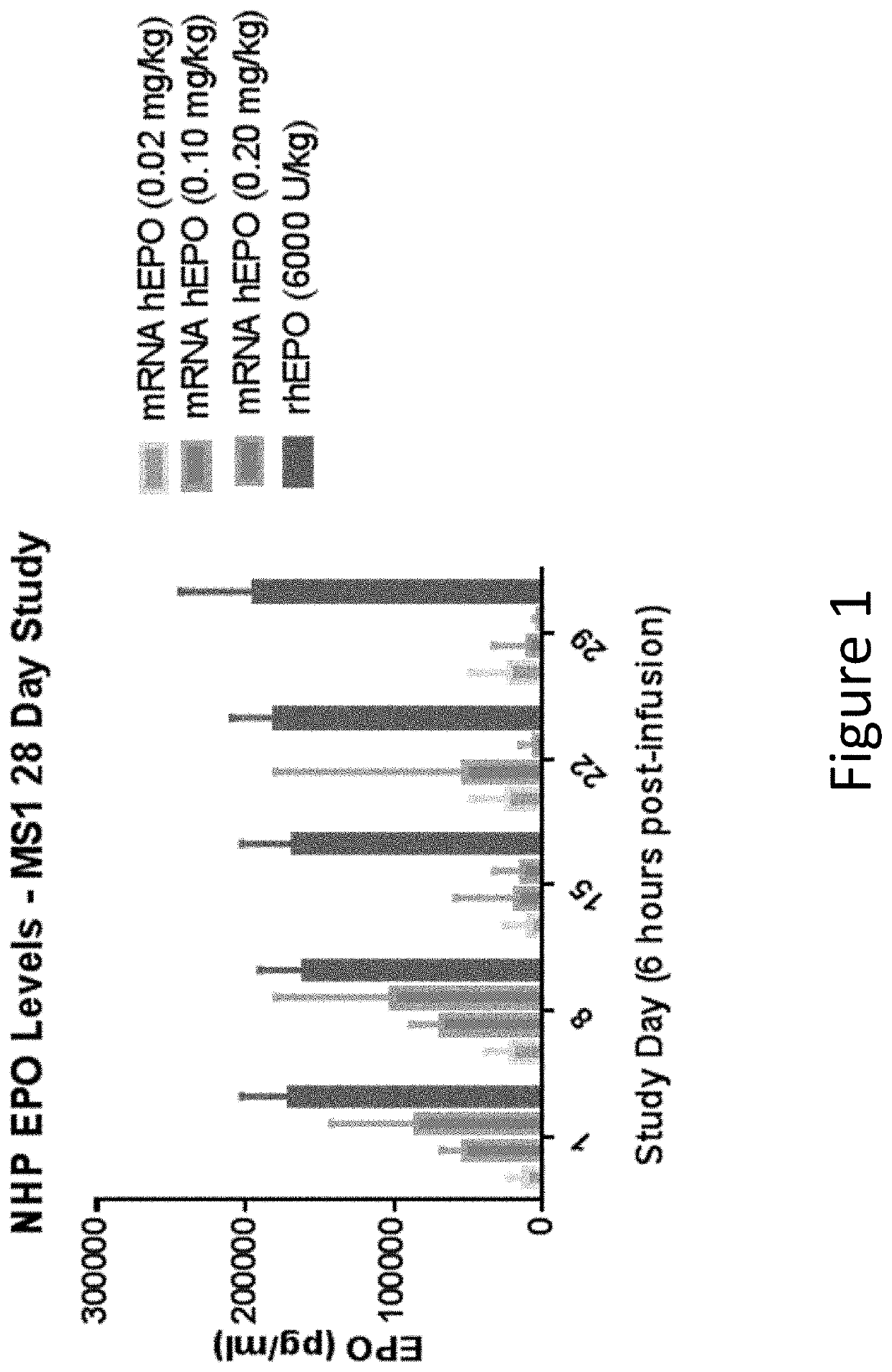
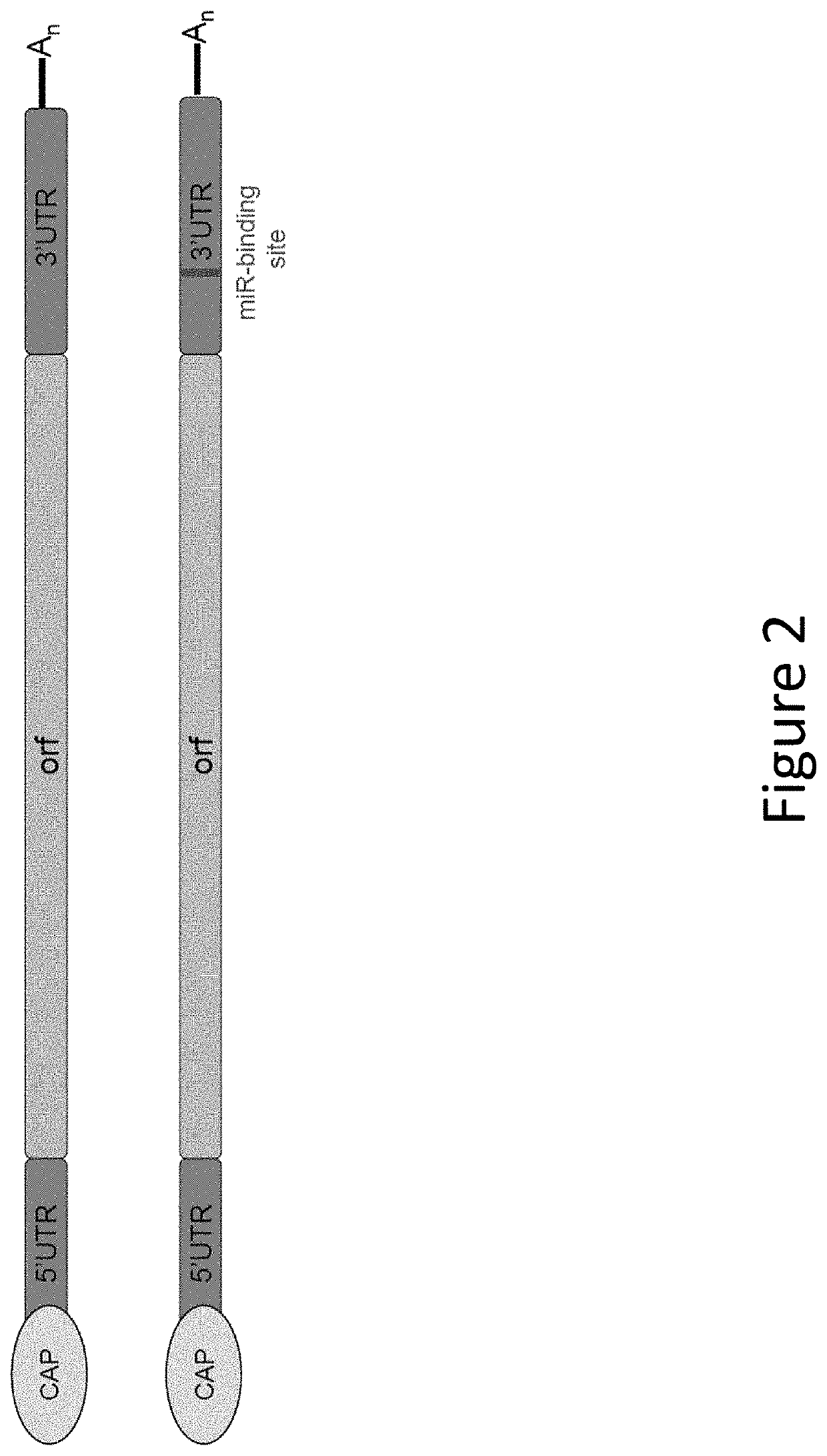
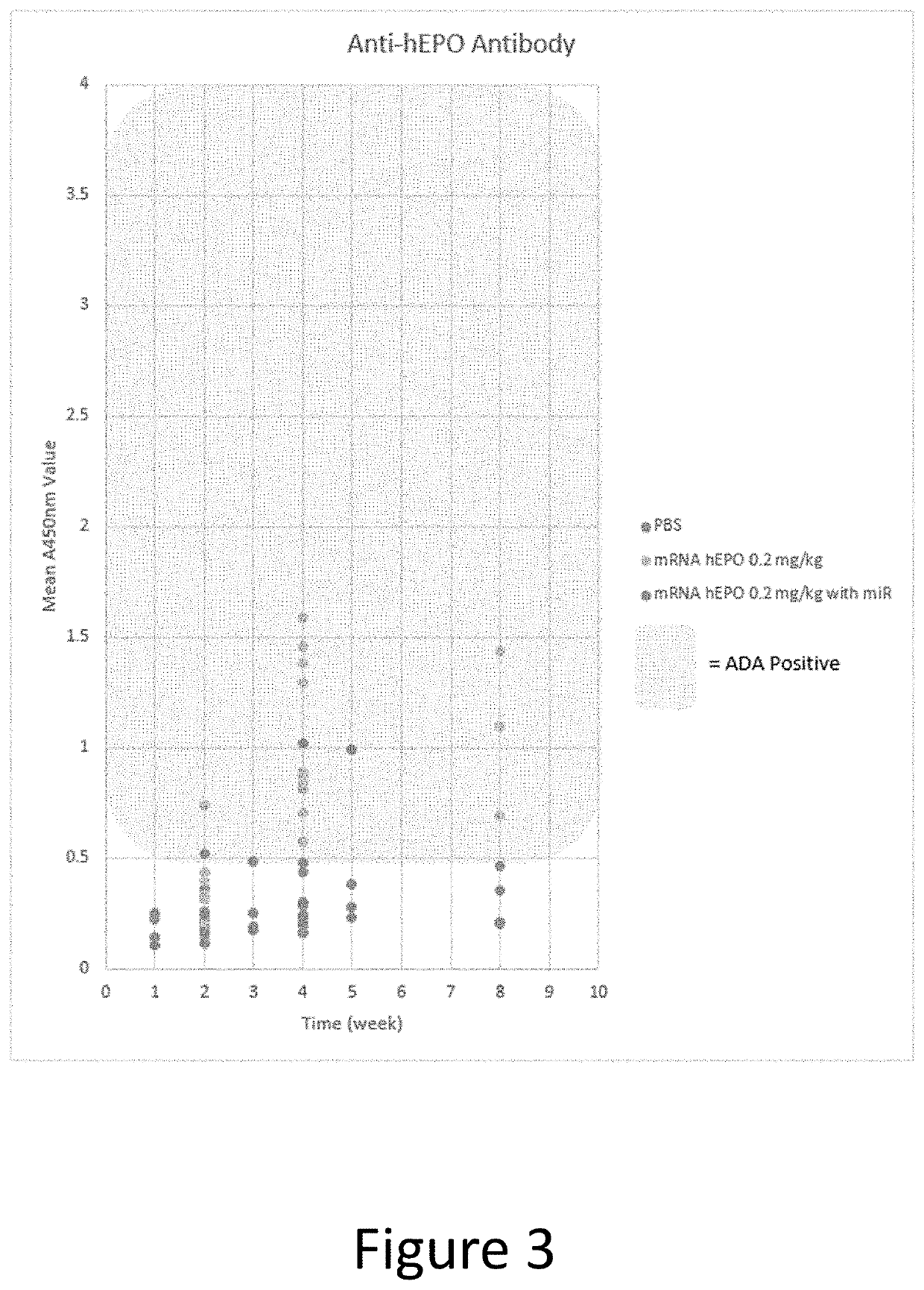
The disclosure features methods of reducing or inhibiting an anti-drug antibody response in a subject, as well as methods of reducing or inhibiting unwanted immune cell activation in a subject to be treated with a messenger RNA (mRNA), comprising administering to the subject a mRNA, e.g., a chemically modified messenger RNA (mmRNA), encoding a polypeptide of interest, wherein the mRNA comprises at least one microRNA (miR) binding site for a miR expressed in immune cells, such as miR-126 binding site and / or miR-142 binding site, such that an anti-drug antibody response to the polypeptide or interest, or unwanted immune cell activation (e.g., B cell activation, cytokine secretion), is reduced or inhibited in the subject. The disclosure further provides therapeutic treatment regimens designed to reduce or inhibit AD A or unwanted immune cell activation (e.g., B cell activation, cytokine secretion) in a subject being treated with mRNA-based therapeutics.
Owner:MODERNATX INC
Anti-cd40 antibodies, uses and methods
ActiveUS20140348836A1Strong binding specificityMinimizing systemic exposureHybrid immunoglobulinsIn-vivo radioactive preparationsDiseaseDendritic cell
The present invention relates to antibodies (and fragments, variants, fusions and derivatives thereof) with multivalent binding specificity for CD40, which have a potency for dendritic cell activation which is higher than, or is equal to, the potency for B cell activation and wherein the antibody, antigen-binding fragment, or fusion, variant or derivative thereof has an affinity (KD) for CD40 of less than 1×10−10 M, which have utility in the treatment of diseases such as cancer. The invention also relates to pharmaceutical compositions, uses, methods and kits comprising such antibodies.
Owner:ALLIGATOR BIOSCI
Compounds containing conjugated allene structure, and pharmaceutical compositions and application thereof
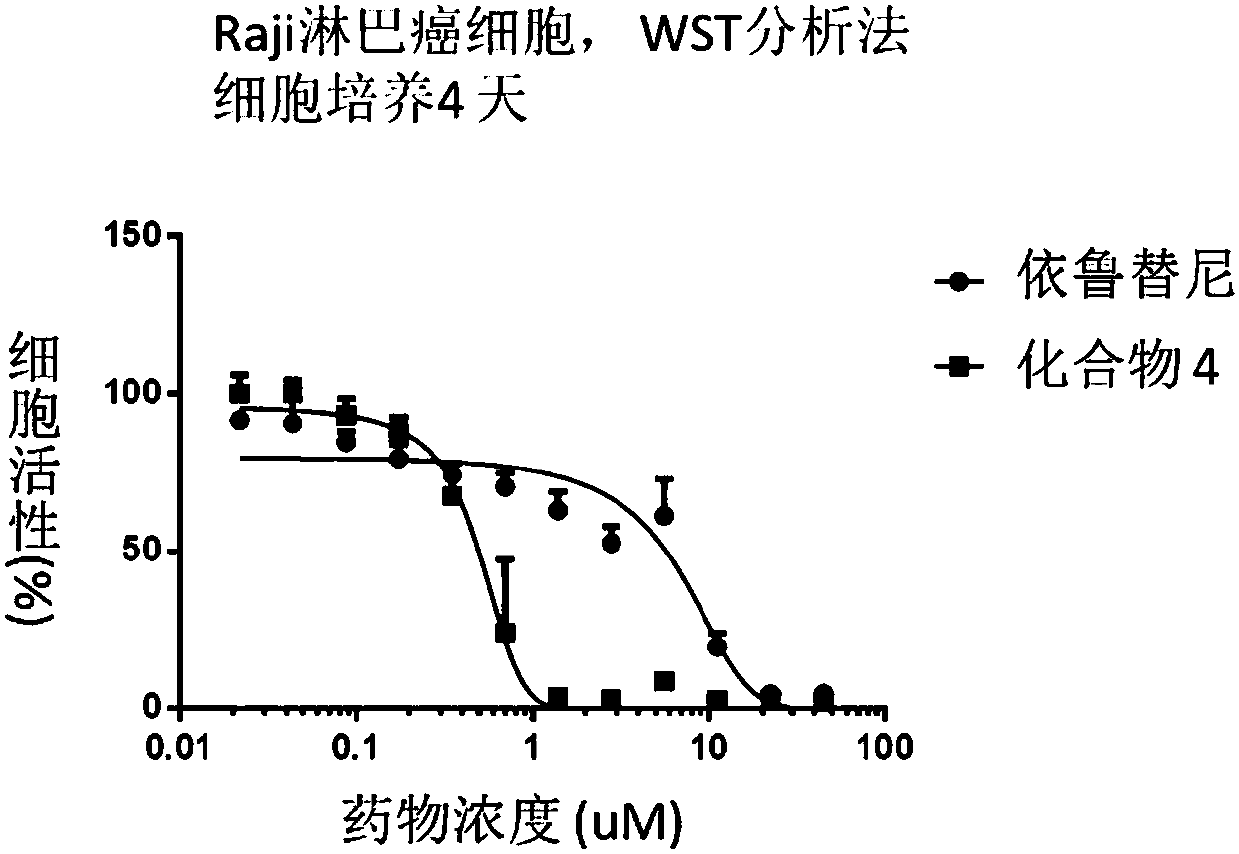
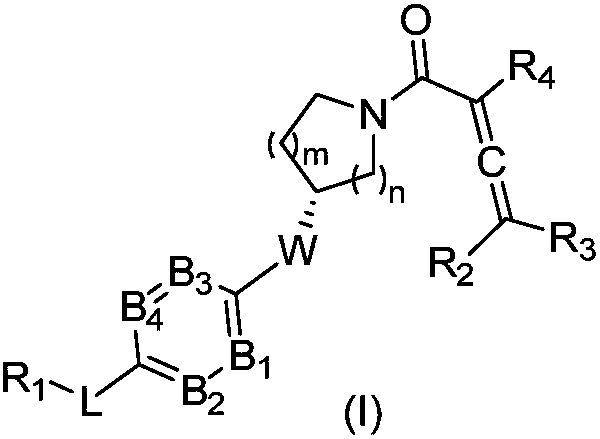
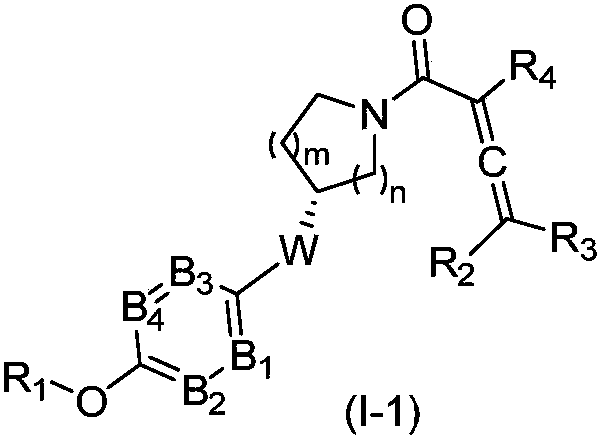
The invention relates to compounds containing conjugated allene structure, and pharmaceutical compositions and application thereof, specifically to compounds as shown in a general formula (I) which are described in the specification or salts thereof, pharmaceutical compositions of the compounds, and application of the compounds as BTK inhibitors and / or B-cell activation inhibitors to prevention ortreatment of abnormal B-cell active activity or diseases related to BTK. The compounds have good lethal effect on cancer cells of lymphoma, breast cancer, liver cancer, intestinal cancer, stomach cancer, lung cancer, cervical cancer and the like, which proves that the compounds have potential of treating related cancers and autoimmune diseases.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Anti-CD40 antibodies and methods of treating cancer
ActiveUS9676862B2Good effectHybrid immunoglobulinsIn-vivo radioactive preparationsDiseaseDendritic cell
The present invention relates to antibodies (and fragments, variants, fusions and derivatives thereof) with multivalent binding specificity for CD40, which have a potency for dendritic cell activation which is higher than, or is equal to, the potency for B cell activation and wherein the antibody, antigen-binding fragment, or fusion, variant or derivative thereof has an affinity (KD) for CD40 of less than 1×10−10 M, which have utility in the treatment of diseases such as cancer. The invention also relates to pharmaceutical compositions, uses, methods and kits comprising such antibodies.
Owner:ALLIGATOR BIOSCI
Methods for inhibition of polyclonal b cell activation and immunoglobulin class switching to pathogenic autoantibodies by blocking cd1-mediated interactions
InactiveUS20110038860A1Slow onsetReduces level of serum IgGBiocidePeptide/protein ingredientsAntigen receptorT-Cell Antigen Receptors
Pathogenic polyclonal B cell activation and immunoglobulin class switching to pathogenic autoantibodies is inhibited by binding molecules that specifically interfere with CD1 antigen, but do not activate signaling (blocking agents), or by molecules that bind to the T cell antigen receptor on T cells that recognize CD1. When CD1 mediated signaling is thus blocked, the T cell response is diminished, resulting in reduced polyclonal B cell activation and reduced immunoglobulin class switching to pathogenic autoantibodies.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Immunomicrosphere for overcoming B cell immunological tolerance and application thereof
InactiveCN102008719APharmaceutical non-active ingredientsGranular deliveryMicrosphereCarrier protein
The invention relates to an immunomicrosphere for overcoming B cell immunological tolerance and application thereof. The immunomicrosphere comprises a microsphere medium, wherein the outside of the microsphere medium is coated with vaccine antigen, and the inside of the microsphere medium is coated with immune carrier protein for activating T cells. The invention also relates to application of the immunomicrosphere for overcoming the B cell immunological tolerance in preparing vaccines and immunological adjuvants. By using the technical scheme, the immunomicrosphere is combined with B cell receptor through the antigen and phagocytized by B cells, and the carrier protein is released and degraded in the cells; the immunomicrosphere is presented to Th cells through B cell MHC II to activate the TH cells to release cell factors, thereby promoting the activation and proliferation of the B cells; and high-titer antibody is generated through the affinity maturation process. The antigen protein and the immune carrier protein are respectively arranged on the surface of the microsphere and inside the microsphere, thereby preventing the antigen protein epitope and the Th cell activation epitope from competing with the B cell receptor.
Owner:SHANGHAI WEIQIU BIOTECH
Method for detecting secretory cells of peripheral blood hepatitis B surface antibody
InactiveCN106802347AShorten detection timeReduce the amount of blood used for testingImmunoassaysHepatitis B surface antibodyPokeweed mitogen
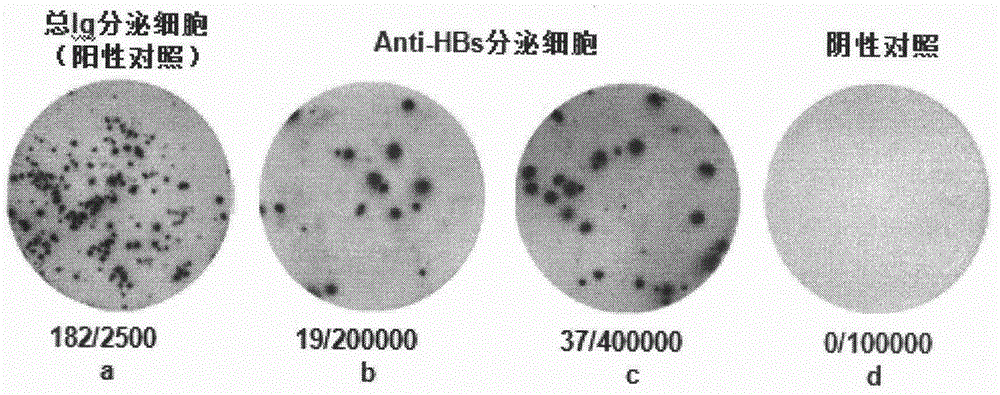
The invention relates to a method for detecting secretory cells of a peripheral blood hepatitis B surface antibody. According to the method disclosed by the invention, human Peripheral Blood Mononuclear Cells (PBMC) are stimulated and activated in vitro, and a pretreated Polyvinylidene Fluoride (PVDF) plate is used for detecting anti-HBs secretory cells in the PBMCs, to detect that a million of PBMCs contain one anti-HBs secretory cell. The method overcomes the detection difficulties in peripheral blood anti-HBs secretory cells, such as small quantity of the peripheral blood anti-HBs secretory cells, low detection rate, low experiment repetitiveness, complex operation, long time consumption and high difficulty, solves the problems that much peripheral blood needs to be adopted and in-vitro B cell activation methods such as CPG, CD40L and pokeweed mitogen are low in activation efficiency, and overcomes the defects of reduction of the detection rate of the anti-HBs secretory cells and the like. According to the method, R848, HBsAg and IL-2 are used for stimulating and activating peripheral blood B cells, and the HBsAg pre-coated PVDF plate is used for capturing the peripheral blood anti-HBs secretory cells, so that the blood amount for detection is reduced, and the detection sensitivity is improved.
Owner:NANJING DRUM TOWER HOSPITAL
Recombinant antigen protein with function of easily activating B cells and preparation method thereof
ActiveCN112679587AEfficient activationImprove activation efficiencyVirus peptidesDepsipeptidesDimerVaccine antigen
The invention relates to a recombinant antigen protein with a function of easily activating B cells and a preparation method thereof. According to the recombinant antigen protein provided by the invention, two or more antigen proteins containing at least one same epitope peptide are linked through a linker peptide to prepare a dimer or polymer recombinant antigen, and the length of the linker peptide enables the distance between the at least two same epitopes to be 3-50 nm. According to the recombinant antigen, at least two of the same epitopes in the antigen protein can be recognized by BCR on B cells at the same time, so that the BCR is cross-linked, and the B cells are more effectively activated. The invention further provides a preparation method of the recombinant protein antigen. The method is universal, can be used for preparing the recombinant antigen with remarkably improved B cell activation efficiency, and can be widely applied to the fields of vaccine antigen preparation, antibody preparation and the like.
Owner:龙湖现代免疫实验室
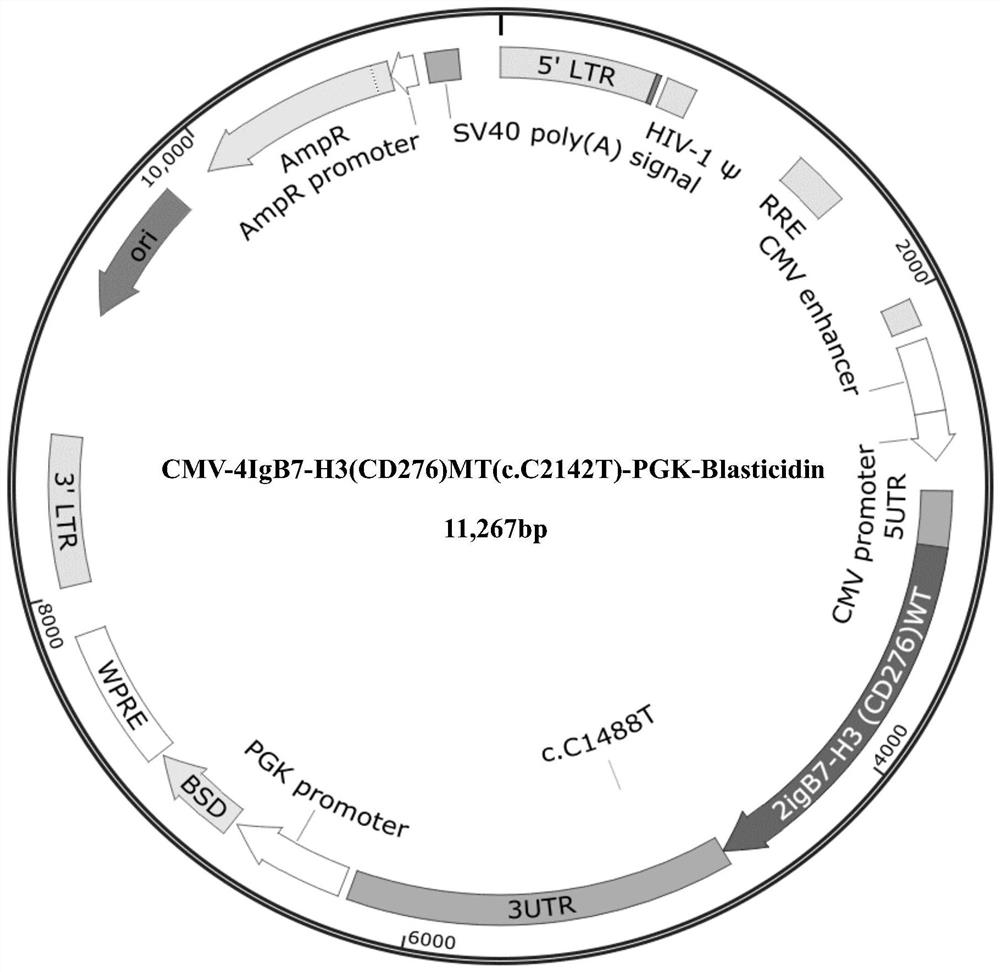
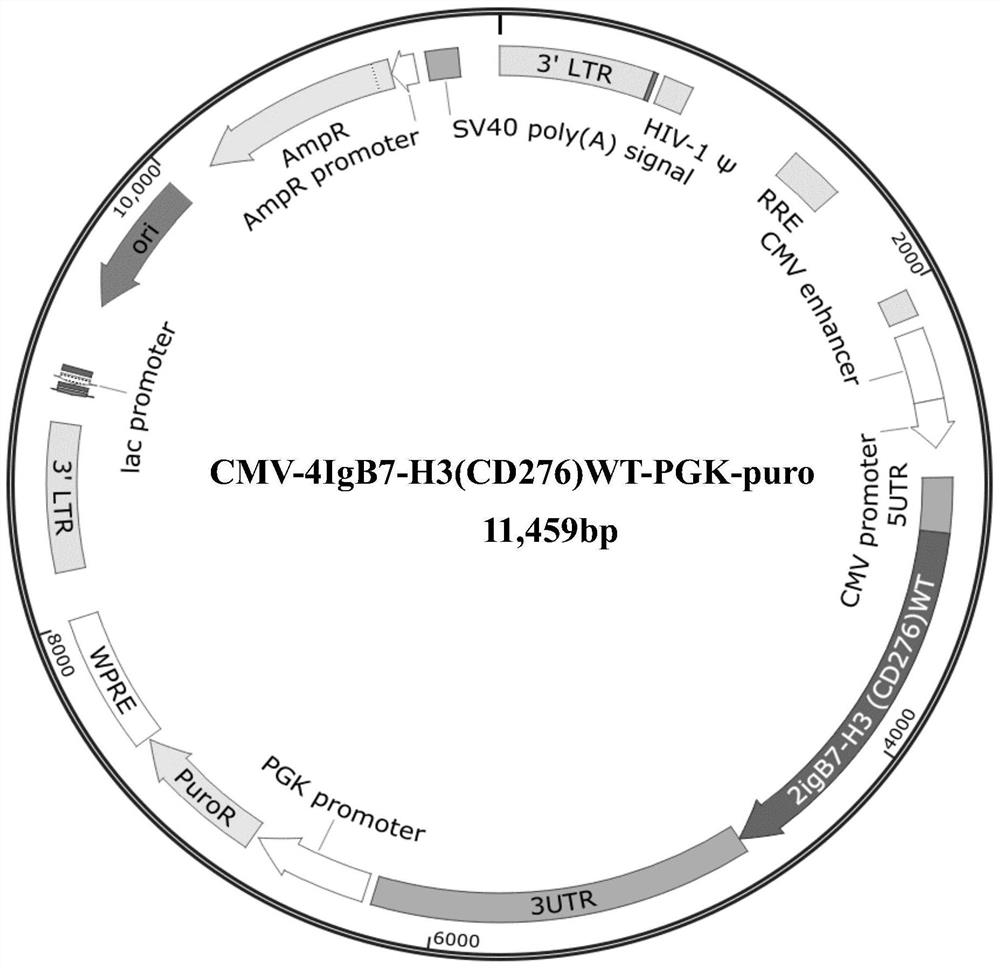
Mutant encoding gene of human 4IgB7-H3 and application thereof in regulating immunity
ActiveCN113151285AStrong biological functionIncrease biological functionPeptide/protein ingredientsNucleic acid vectorDiseaseDendritic cell
The invention discloses a new mutant encoding gene of human 4IgB7-H3 and application thereof in regulating immunity. The nucleotide sequence thereof is shown in SEQ ID NO:1. The mutant encoding gene of human 4IgB7-H3 has an enhanced biological function as compared to the wild-type gene, that is, the binding of the mutant encoding 4IgB7-H3 with an inhibitory receptor has an enhanced inhibitory effect on the proliferation and activation of T cells. 4IgB7-H3, as a costimulatory molecule, plays an important role in the processes of T and B cell activation, helper T cell differentiation, signal transduction and cytokine secretion of effector cells. The mutant encoding gene of human 4IgB7-H3 plays a role in solving and treating the main reason why it is difficult to induce tumor specific T cell activation in tumor escape from immune surveillance, and has prominent and wide application prospects in preparation of genomic engineering, gene recombination, immunotherapeutic drugs, vaccines and protein substitute therapies for treating malignant tumors and infectious diseases based on T cells and dendritic cells, allergy relief and the like.
Owner:白素梅
Cloning of chicken CR2 gene, expression and purification of protein as well as preparation of polyclonal antibody of protein
InactiveCN110423271AHigh preparation titerHigh potencyCell receptors/surface-antigens/surface-determinantsSerum immunoglobulinsEscherichia coliCell signaling
The invention provides cloning of chicken CR2 gene, expression and purification of protein as well as preparation of a polyclonal antibody of protein. An amino acid sequence and a gene sequence of ChCR2 protein are provided, a ChCR2 prokaryotic expression vector containing different labels is constructed, an Escherichia coli prokaryotic expression system is used for expressing ChCR2 protein, and the protein is purified. The purified protein can prepare the polyclonal antibody with higher titer. The ChCR2 gene plays important roles in a chicken immune system. CR2 can link congenital complementmedicated immune response with pathogens and foreign antigens and combine with C3d covalently attached to a target to produce adaptive immune response, and caused cell signaling phenomenon can greatlyreduce threshold of B cell activation. B cells are important in the immune system, so that the invention finds that ChCR2 is of great significance in both fundamental research and clinical application.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Monoclonal antibody MR1 and uses thereof
InactiveUS20060008460A1Peptide/protein ingredientsNGF/TNF-superfamilyAutoimmune conditionCellular antigens
The present invention relates to a counter-receptor, termed CD40CR, for the CD40 B-cell antigen, and to soluble ligands for this receptor, including fusion molecules comprising at least a portion of CD40 protein. It is based, at least in part, on the discovery that a soluble CD40 / immunoglobulin fusion protein was able to inhibit helper T-cell mediated B-cell activation by binding to a novel 39 kD protein receptor on helper T-cell membranes. The present invention provides for a substantially purified CD40CR receptor; for soluble ligands of CD40CR, including antibodies as well as fusion molecules comprising at least a portion of CD40 protein; and for methods of controlling B-cell activation which may be especially useful in the treatment of allergy or autoimmune disease.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Novel method for producing antibodies
ActiveCN111094552AImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsAntigenPeripheral blood mononuclear cell
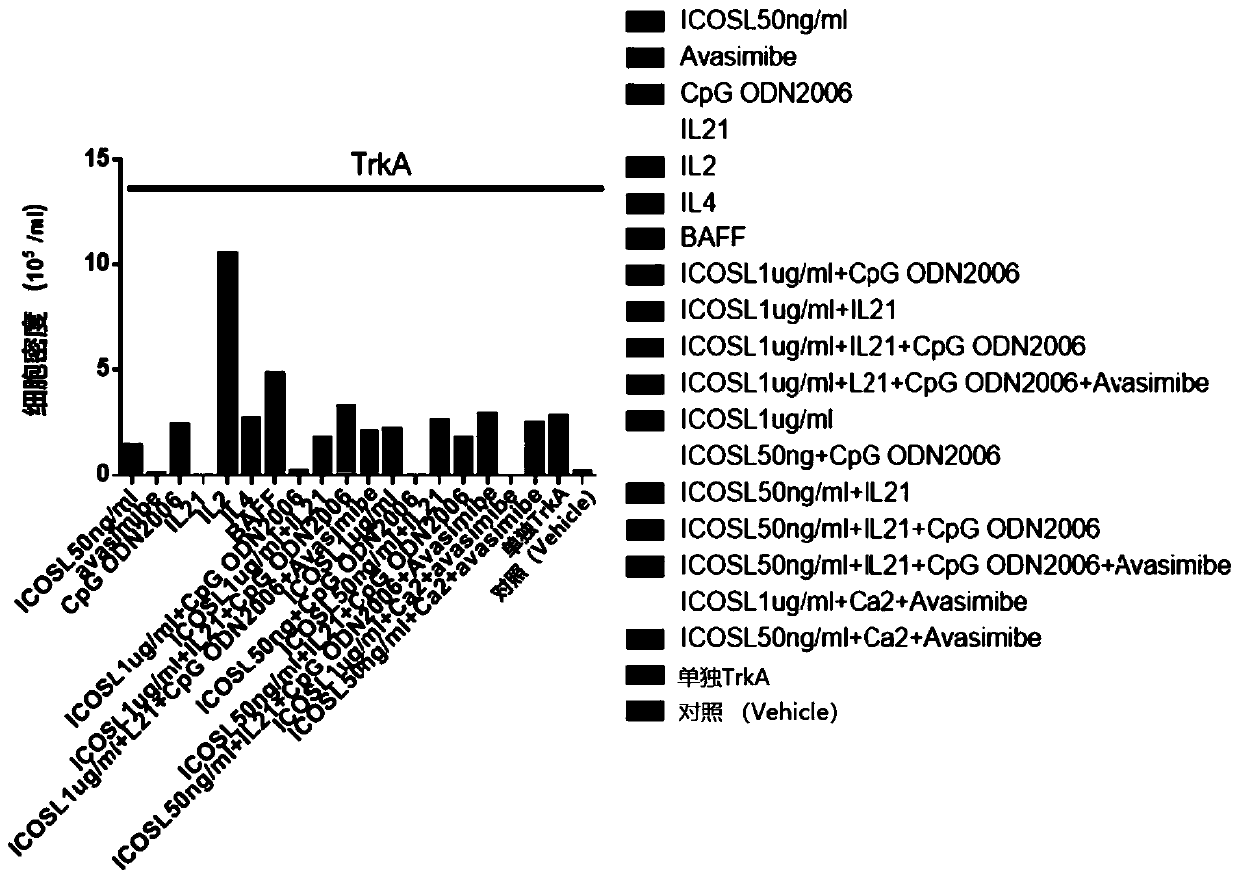
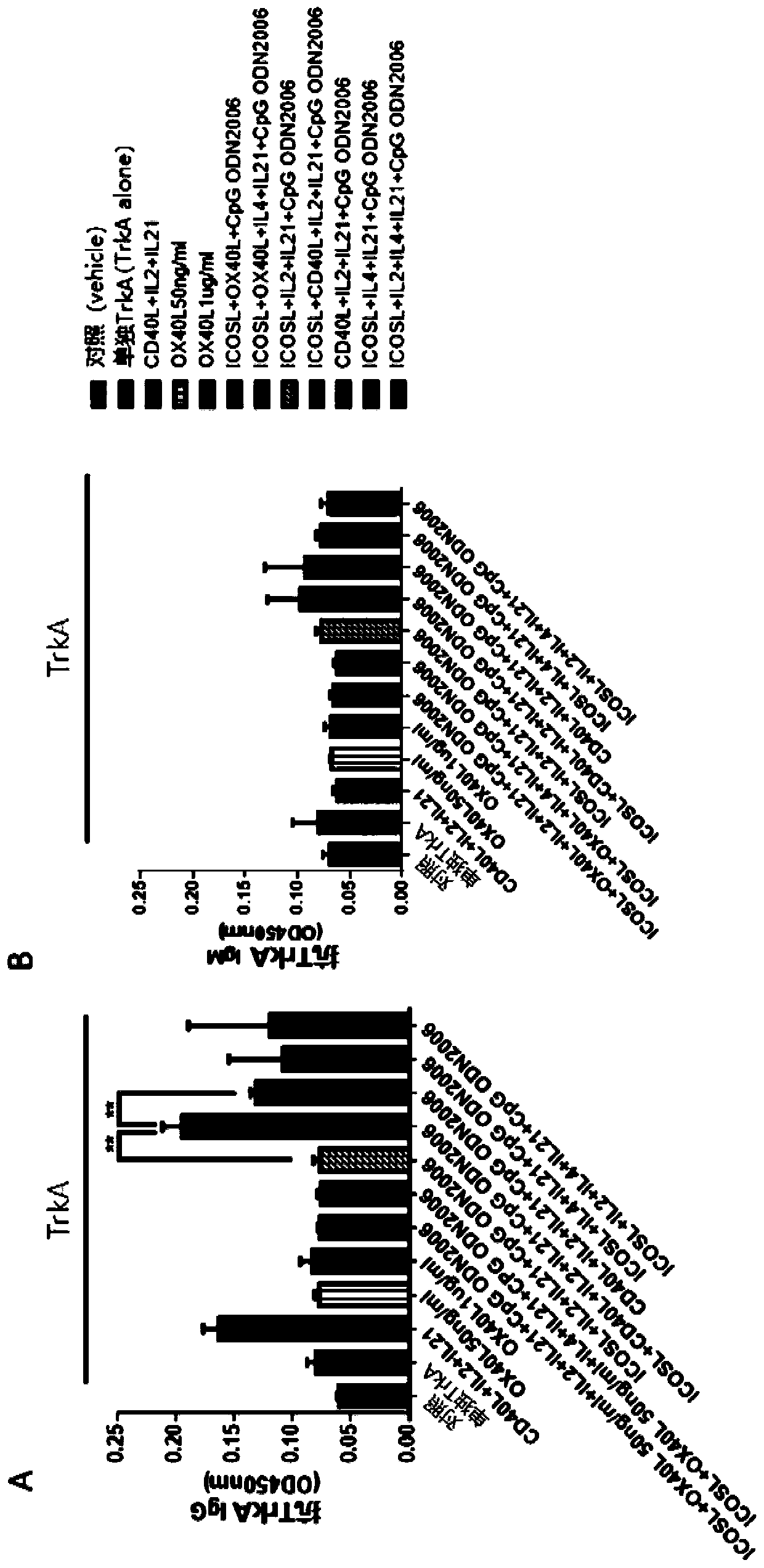
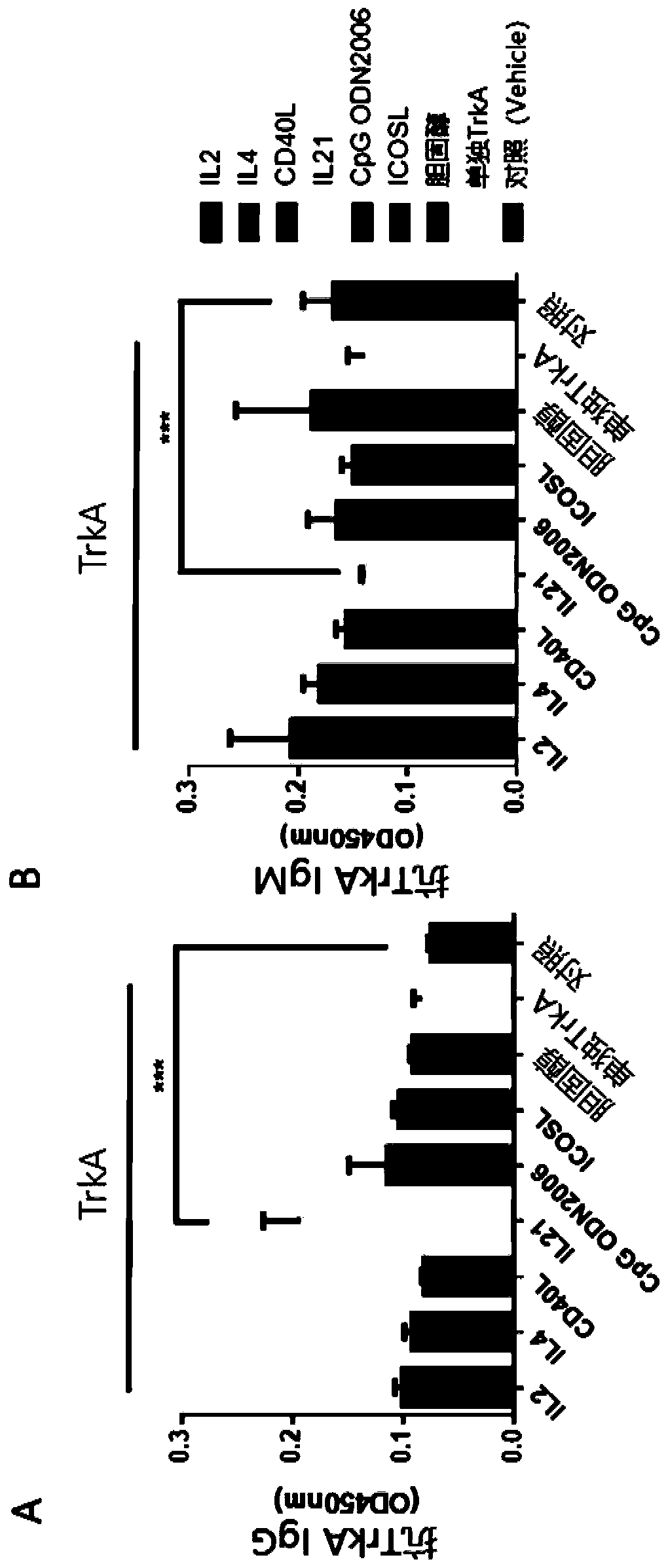
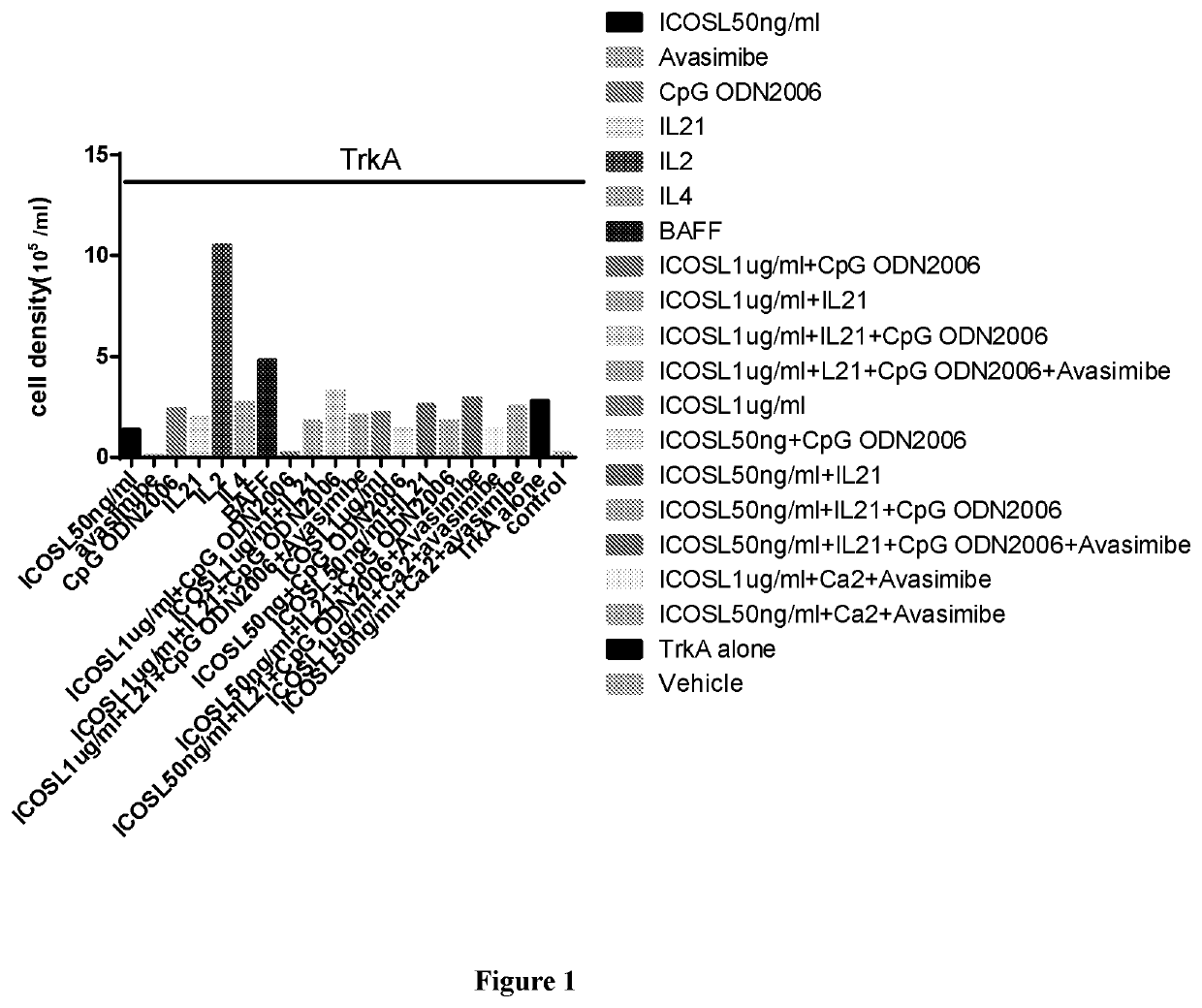
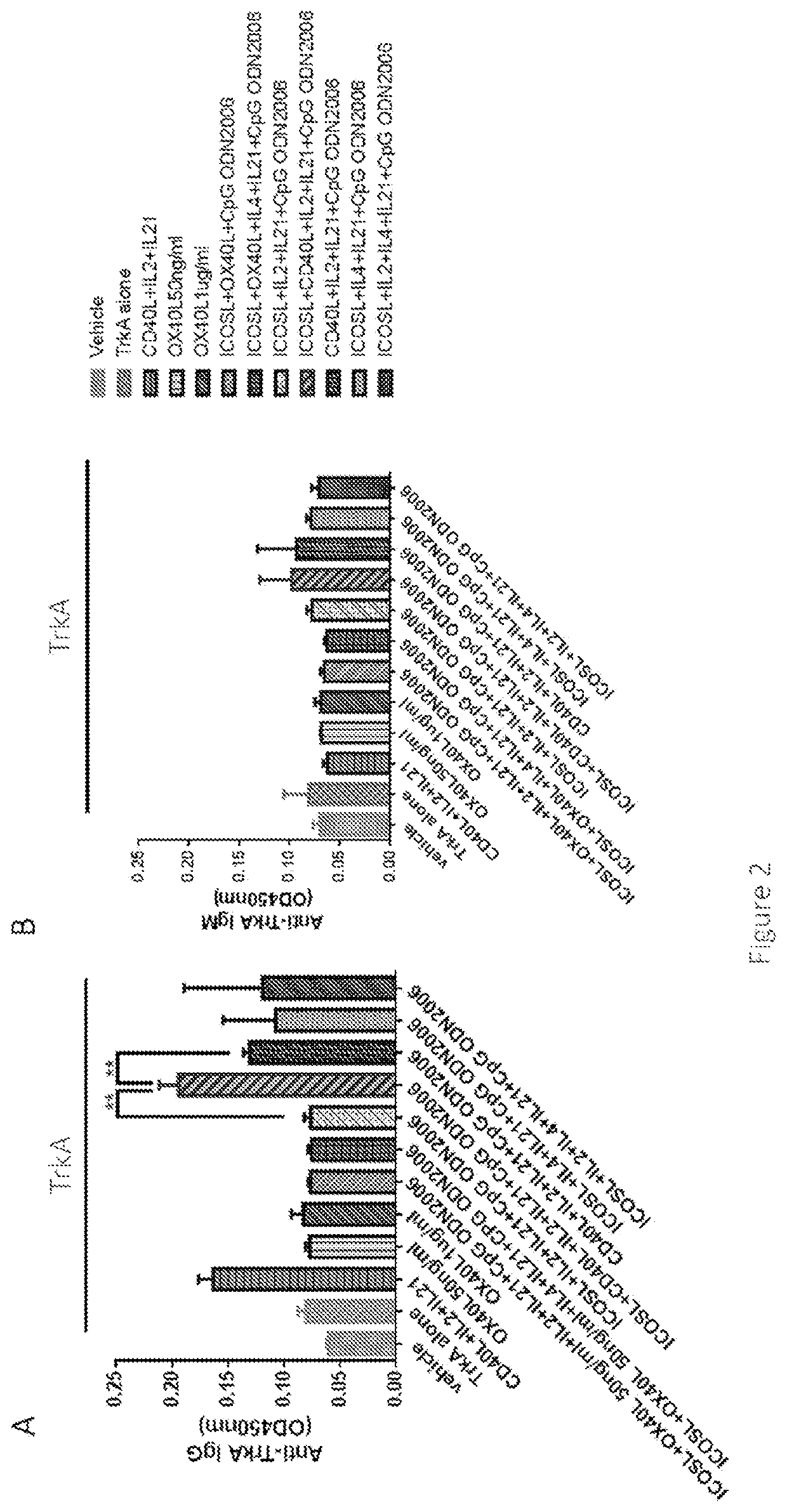
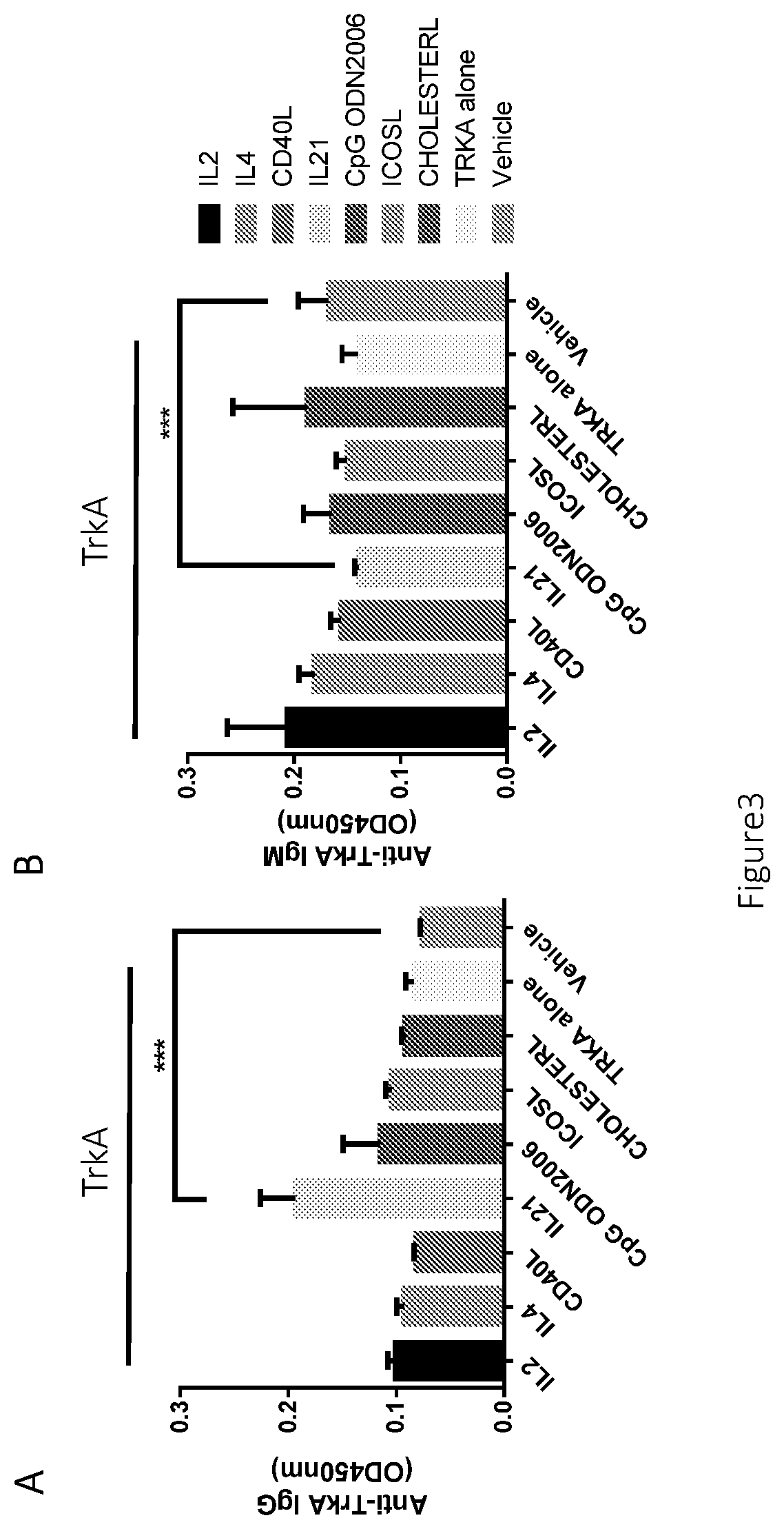
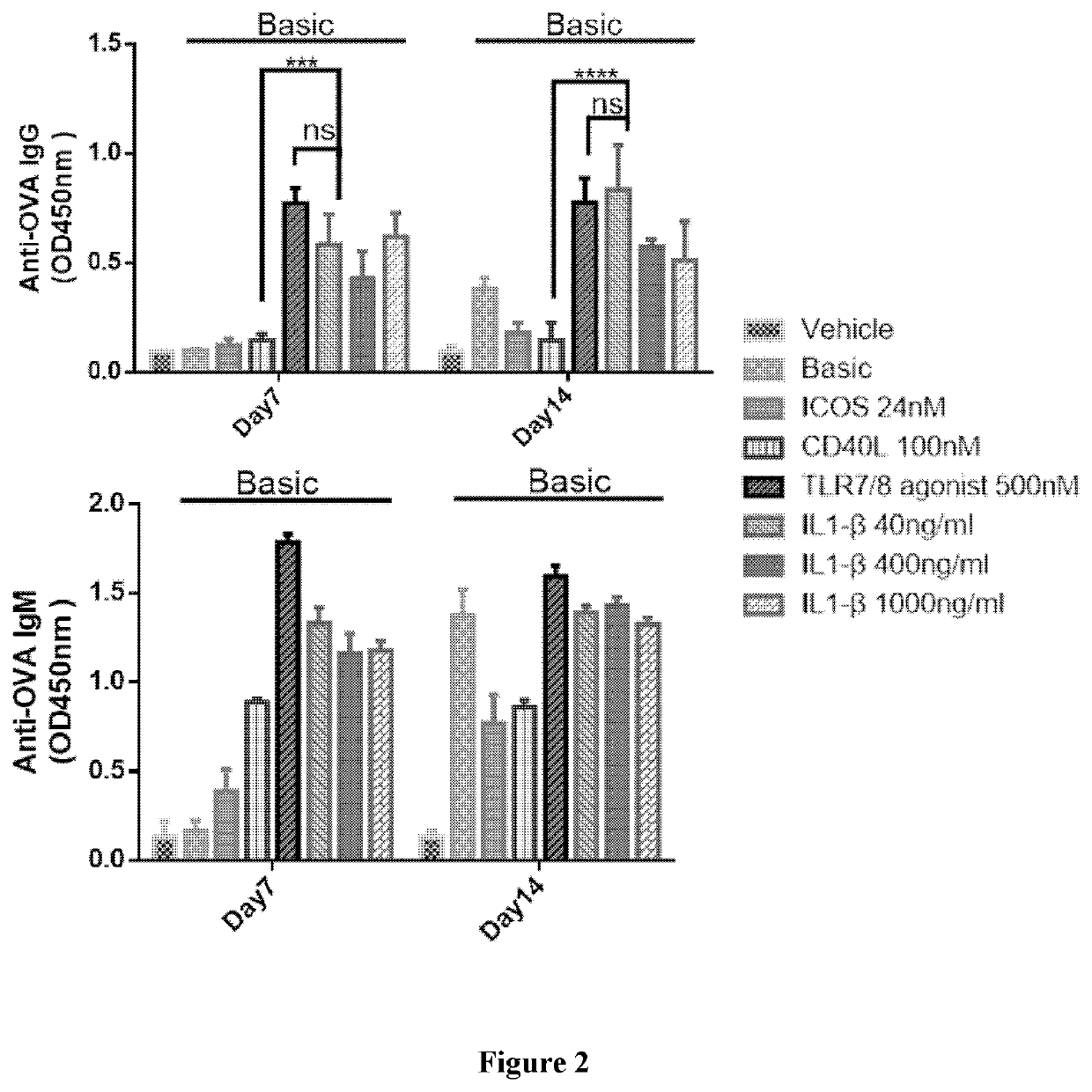
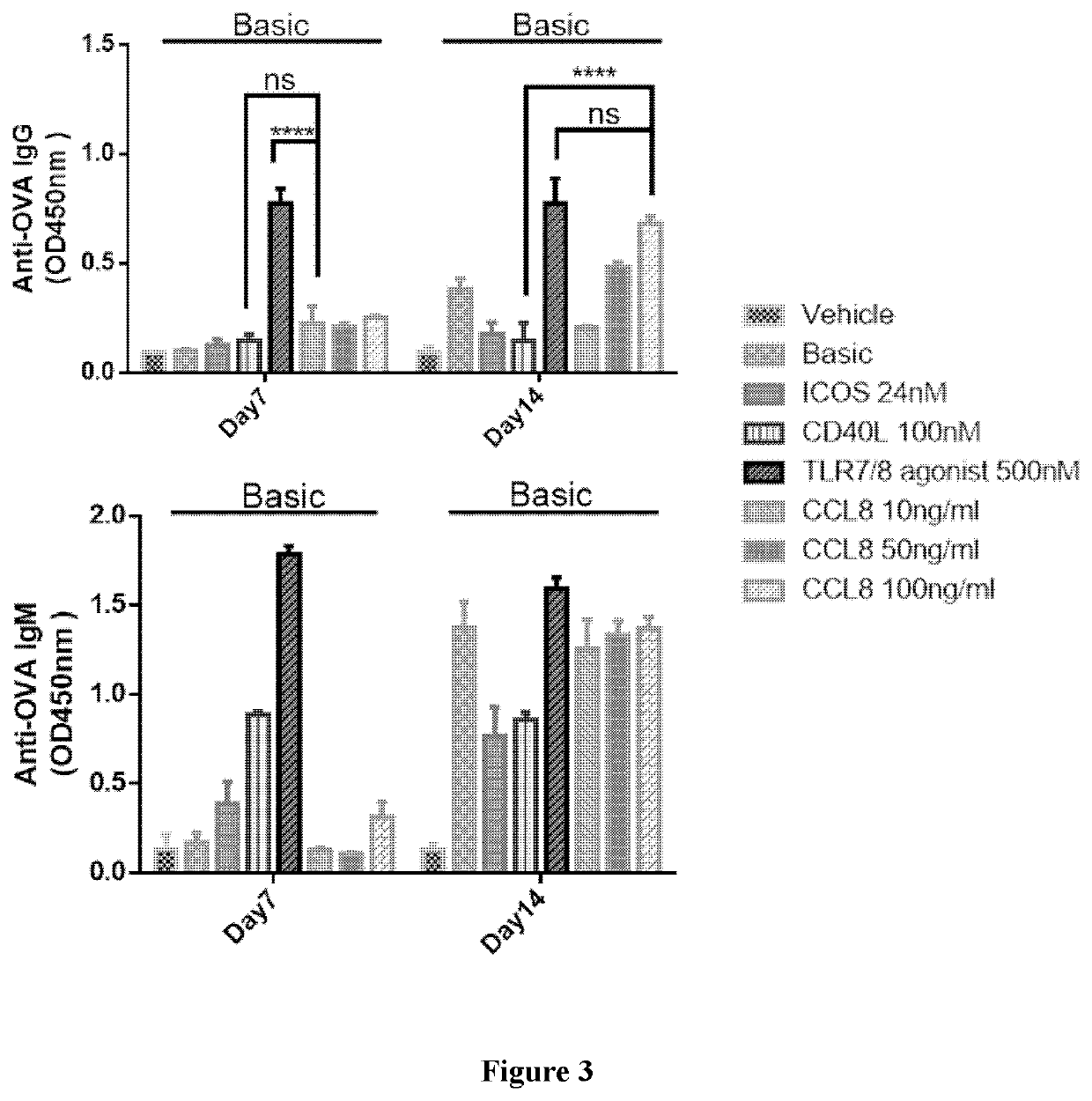
Disclosed is a method for producing an antibody or an antigen-binding fragment thereof comprising a step of cultivating PBMCs in a medium comprising CD40L, ICOSL, ICOS, and / or TLR agonist. Also provided herein is a method for inducing proliferation of PBMCs, B cell activation and differentiation, and / or B cell maturation, comprising a step of cultivating PBMCs in a medium comprising IL2. Also provided herein is a method for promoting class switch in an antibody-producing PBMC to produce IgG, comprising a step of cultivating the antibody-producing PBMC in a medium comprising IL21.
Owner:TSINGHUA UNIV
Catalytic antibodies and a method of producing same
InactiveUS20090269355A1Avoid interactionSugar derivativesAntibody mimetics/scaffoldsAdjuvantCatalytic antibody
The present invention relates generally to a growth factor precursor and its use to select production of antigen specific catalytic antibodies. Such catalytic antibodies are produced following B cell activation and proliferation induced by catalytic cleavage products of a target antigen portion of the growth factor precursor of the present invention. A particularly useful form of the growth factor precursor is as a nucleic acid vaccine. The nucleic acid vaccine of the present invention preferably further comprises a molecular adjuvant. Another aspect of the present invention comprises a growth factor precursor in multimeric form. The growth factor precursor of the present invention is useful for generating catalytic antibodies for both therapeutic, diagnostic and industrial purposes.
Owner:KOENTGEN FRANK
Compositions and methods for treating disease states associated with activated t cells and/or b cells
Disclosed are combination therapies and related compositions that may contain one or more of a p53 potentiating agent, a DNA-damaging agent, an agent that inhibits cell cycle check point, and a pharmaceutically acceptable carrier. Also disclosed are methods of using such compositions for the treatment of conditions related to T cell and / or B cell activation in subjects in need of such treatment.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Method for treating cancer
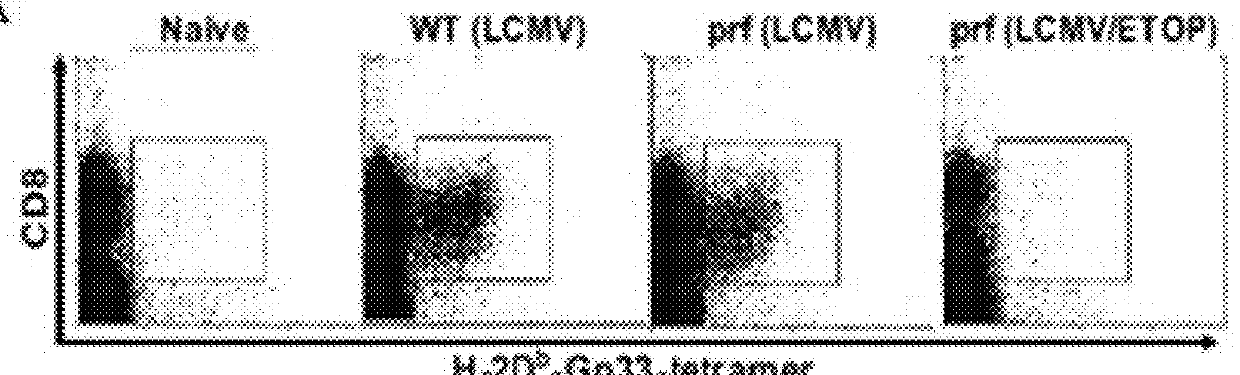
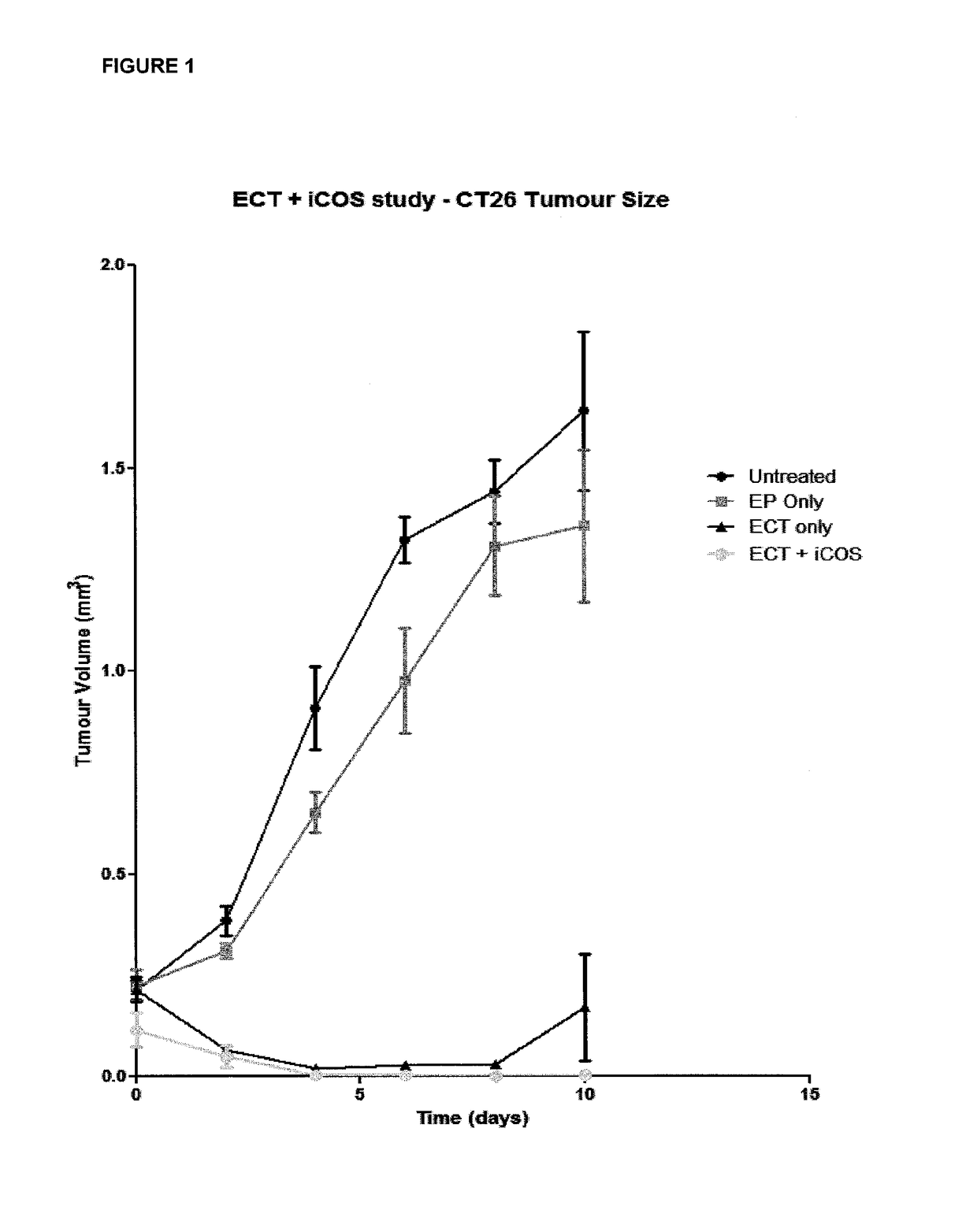
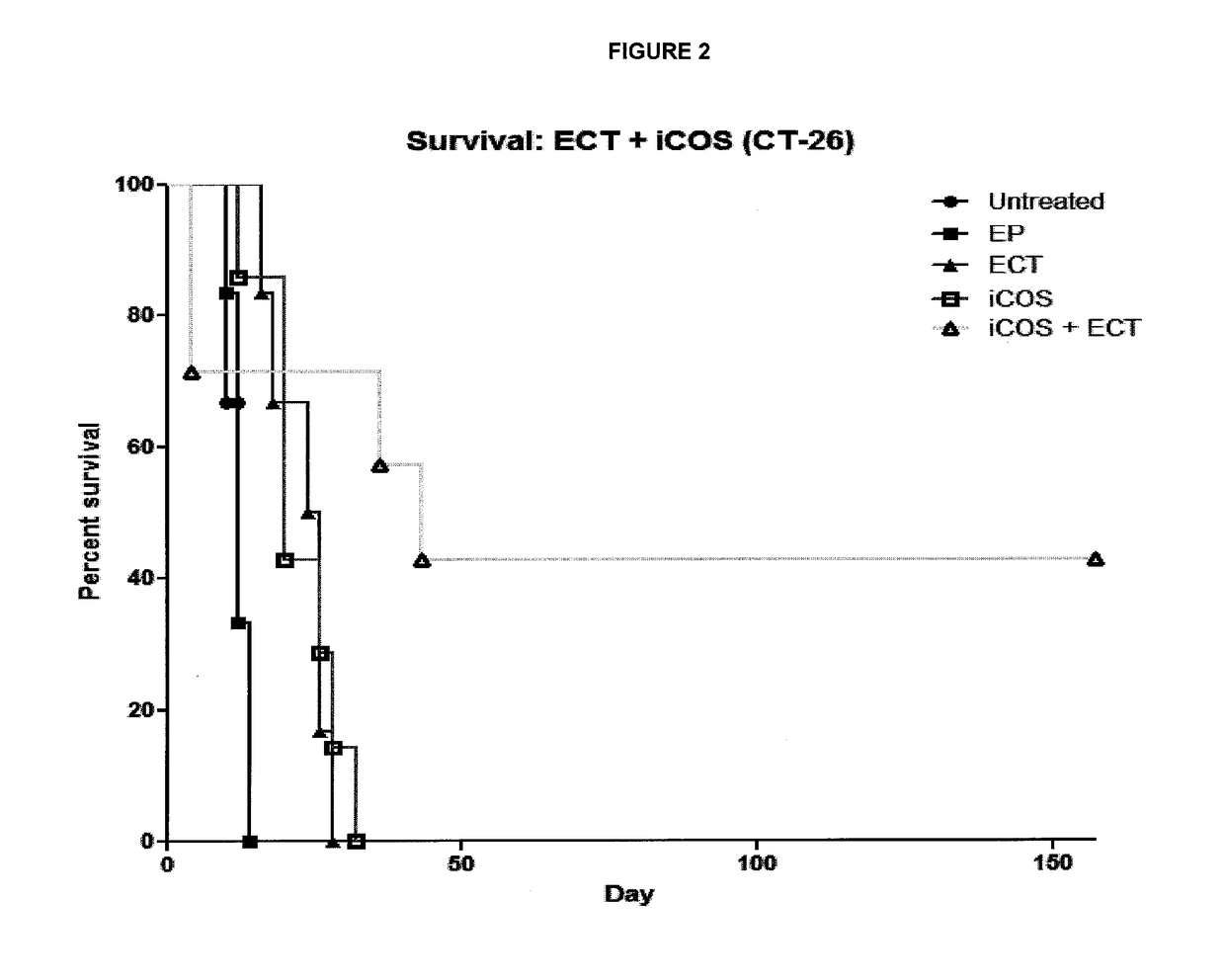
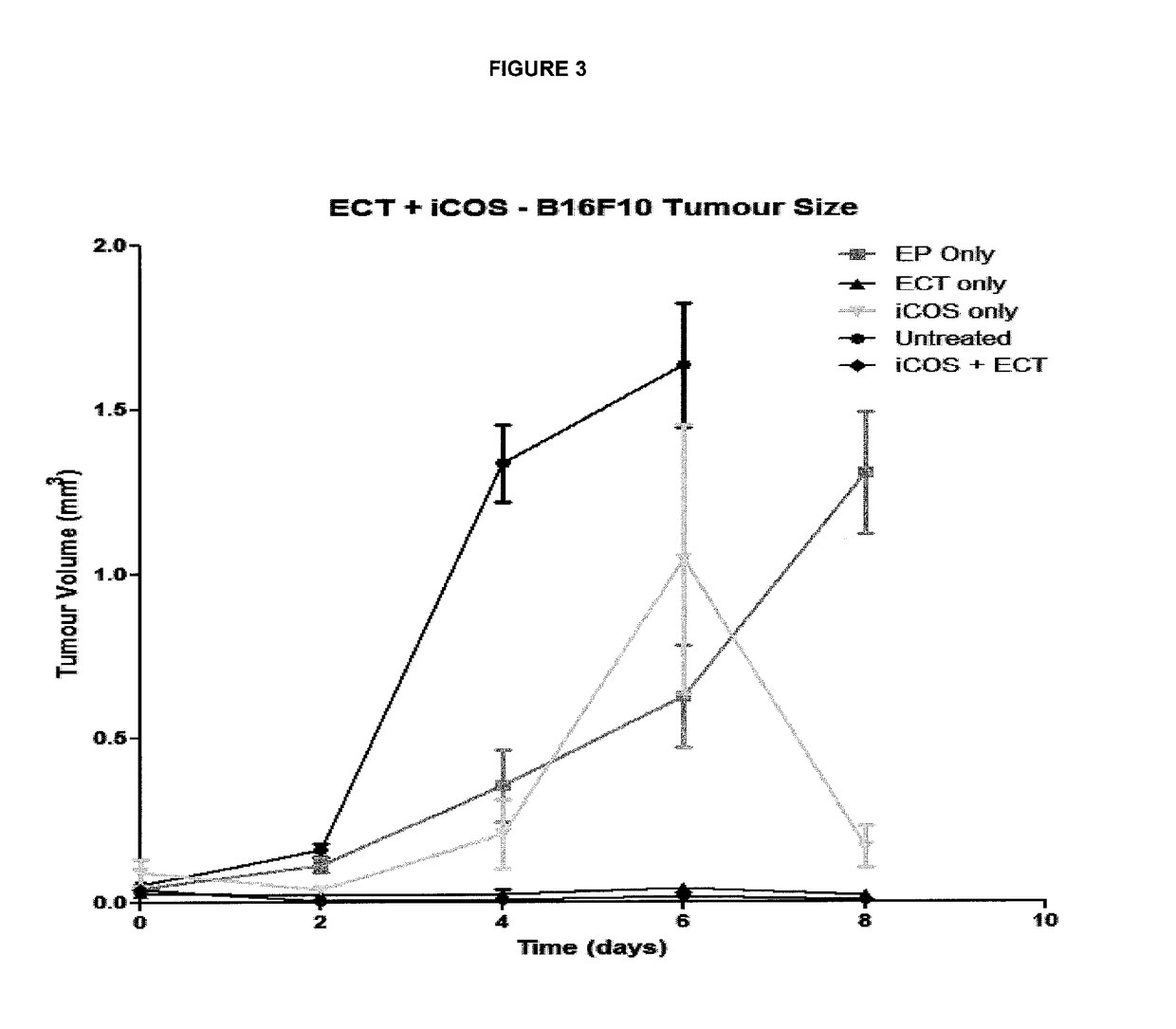
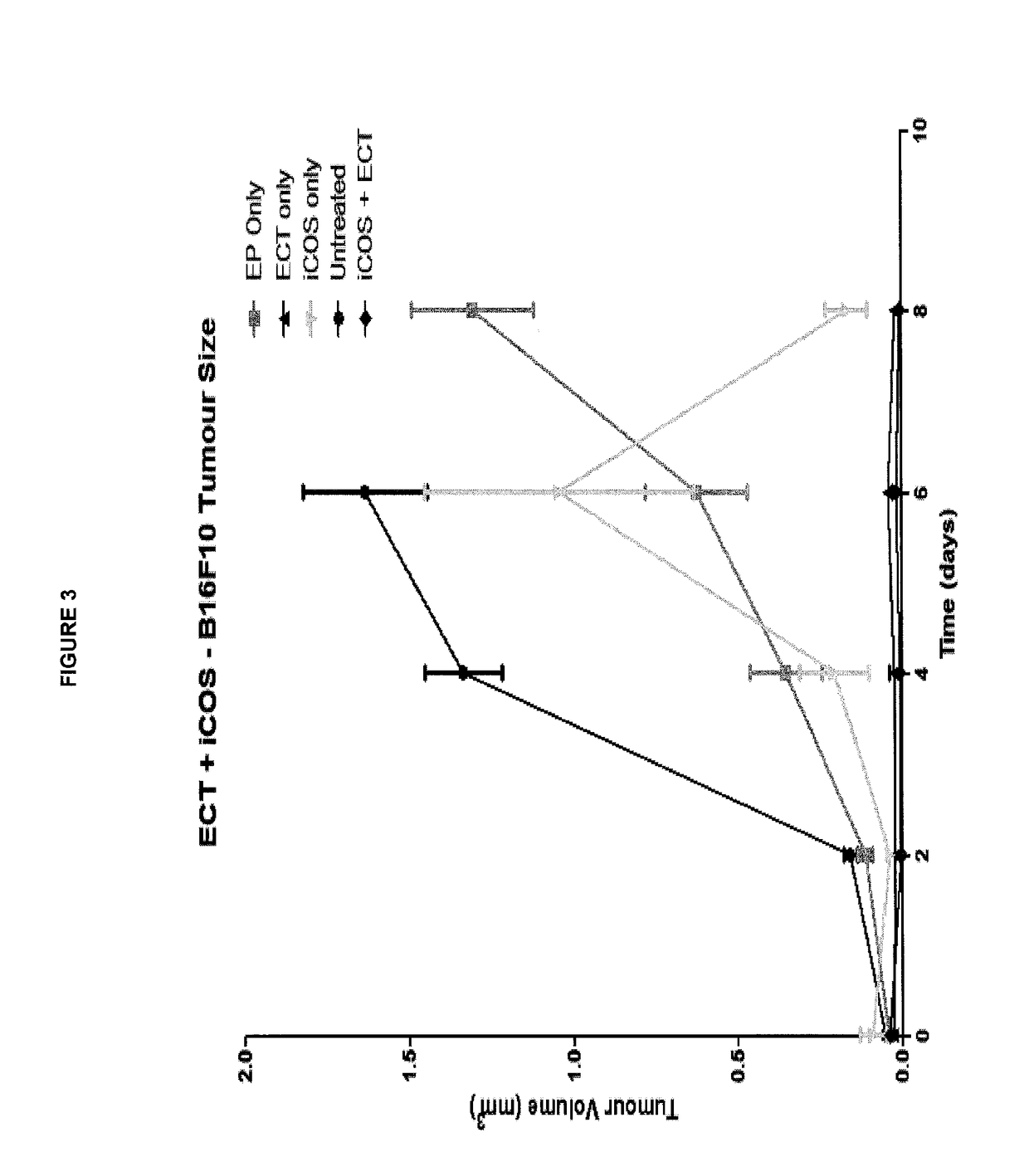
ActiveUS20170182164A1Decreasing interstitial pressurePromote activationSonopheresisPeptide/protein ingredientsB-cell activationElectroporation
The present invention relates to a method for the treatment of cancer in a subject which comprises the steps of: (i) administering electroporation to a tumour in the subject; and (ii) administering a T or B-cell activating agent to the subject, wherein step (i) and (ii) may be performed in either order.
Owner:UNIV COLLEGE CORK NAT UNIV OF IRELAND CORK
Novel B cell activation model for specific antigen loading and induction of CTL
InactiveCN103789261AAvoid the defects of long time culture and low efficiency of culture expansionReliable and Simple Cell ExperimentsBlood/immune system cellsCD20Peripheral blood mononuclear cell
The invention relates to a novel B cell activation model for specific antigen loading and induction of CTL, which comprises the steps of separating human peripheral blood to obtain peripheral blood mononuclear cells, separating the B cells therein by use of CD20 magnetic beads, and culturing in an incubator; removing PBMC of the B cells; after culturing the B cells, loading the HBcAg18-27 polypeptide, wherein 50mu g / ml polypeptide is added, and the loading time is 12-18 hours; after antigen loading, washing the B cells and adding into the PBMC cell without B cells, wherein the ratio of the B cells to T cells is 1:10; continuously culturing for 4 days and taking out the cultured cells; detecting the HBV HBcAg18-27 polypeptide specific CTL cells with the pentamer technology. By adopting the B cell activation model provided by the invention, the shortcomings that the content of the peripheral blood mononuclear cells is lower than 1%, the amplification efficiency is not high, the culture time is long, the culture cost is high and the like are overcome; a reliable and simple cell experiment model is established, and the defects that the dendritic cells need long-time culture and the culture amplification efficiency is low are avoided.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
Anti-CD40 antibodies capable of blocking B-cell activation
Methods for preventing or treating an antibody-mediated disease in a patient are presented, the methods comprising administration of a monoclonal antibody capable of binding to a human CD40 antigen located on the surface of a human B cell, wherein the binding of the antibody to the CD40 antigen prevents the growth or differentiation of the B cell. Monoclonal antibodies useful in these methods, and epitopes immunoreactive with such monoclonal antibodies are also presented.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Cloning of chicken CR2 gene, expression and purification of protein and preparation of polyclonal antibody of chicken CR2 gene
InactiveCN111763256AEnriched ImmunologyHigh preparation titerCell receptors/surface-antigens/surface-determinantsSerum immunoglobulinsEscherichia coliEnterobacter
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
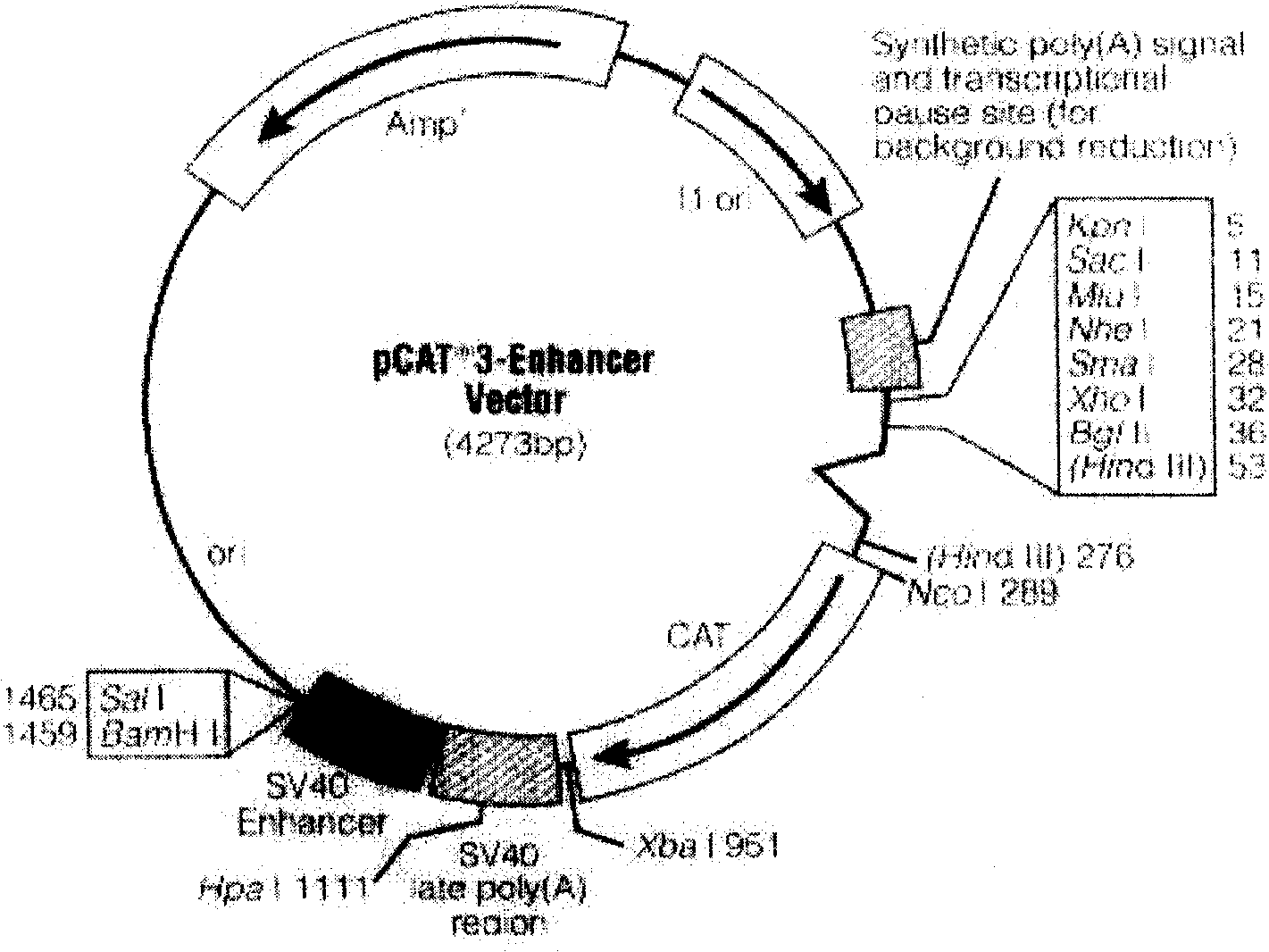
Recombinant plasmids containing B cell activation factor gene promoters with different lengths and their preparation method and application
InactiveCN100554430CRecombinant plasmids are reliableGenetic material ingredientsImmunological disordersAntagonismPromoter
The invention relates to the technical field of biomedical engineering. At present, the structure, function analysis and transcriptional regulation of the B cell activating factor (BAFF) gene promoter are still blank. The present invention provides five recombinant plasmids containing different lengths of the B cell activating factor (BAFF) gene promoter. It consists of -1349~-1099bp, -1349~-893bp, -1349~-743bp, -1349~-431bp, -1349~-329bp fragments of the upstream promoter sequence of human BAFF gene and without promoter, but containing chloramphenicol acetyl The carrier composition of the base transferase CAT reporter gene, the invention also provides the preparation method and application of the recombinant plasmid. The recombinant plasmids constructed by the present invention containing BAFF gene promoters of different lengths are accurate and reliable, and have laid a foundation for further research on the transcriptional regulation mechanism of human BAFF gene activation; and the method of comparing the transcriptional activity of recombinant promoters with reporter gene activity is simple and can be quantified , is a simple method for screening drugs for antagonism / treatment of SLE at the transcriptional level, and also provides a new target for exploring the prevention and treatment of SLE.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Novel method for producing antibodies
PendingUS20200172615A1Enhance antibody productionIncrease productionImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsAntigenTlr agonists
Disclosed is a method for producing an antibody or an antigen-binding fragment thereof comprising a step of cultivating PBMCs in a medium comprising CD40L, ICOSL, ICOS, and / or TLR agonist. Also provided herein is a method for inducing proliferation of PBMCs, B cell activation and differentiation, and / or B cell maturation, comprising a step of cultivating PBMCs in a medium comprising IL2. Also provided herein is a method for promoting class switch in an antibody-producing PBMC to produce IgG, comprising a step of cultivating the antibody-producing PBMC in a medium comprising IL21.
Owner:TSINGHUA UNIV
Methods for therapeutic administration of messenger ribonucleic acid drugs
ActiveUS20210260097A1Dramatic inhibitionReducing or inhibiting anti-drug antibody responsesOrganic active ingredientsNucleic acid vectorAntiendomysial antibodiesBinding site
The disclosure features methods of reducing or inhibiting an anti-drug antibody response in a subject, as well as methods of reducing or inhibiting unwanted immune cell activation in a subject to be treated with a messenger RNA (mRNA), comprising administering to the subject a mRNA, e.g., a chemically modified messenger RNA (mmRNA), encoding a polypeptide of interest, wherein the mRNA comprises at least one microRNA (miR) binding site for a miR expressed in immune cells, such as miR-126 binding site and / or miR-142 binding site, such that an anti-drug antibody response to the polypeptide or interest, or unwanted immune cell activation (e.g., B cell activation, cytokine secretion), is reduced or inhibited in the subject. The disclosure further provides therapeutic treatment regimens designed to reduce or inhibit ADA or unwanted immune cell activation (e.g., B cell activation, cytokine secretion) in a subject being treated with mRNA-based therapeutics.
Owner:MODERNATX INC
Method for treating cancer
ActiveUS10143750B2Promote activationGenerate immune responseSonopheresisPeptide/protein ingredientsB-cell activationElectroporation
Owner:UNIV COLLEGE CORK NAT UNIV OF IRELAND CORK
Recombinant plasmid containing effective B cell activation factor gene promotor and its preparation method and uses
InactiveCN100457911CThe method of measuring activity and comparative activity is simpleRecombinant plasmids are reliableGenetic material ingredientsImmunological disordersFactor iiB-cell activation
The invention discloses a recombination plasmid with effective BAFF gene promotor in the biomedical engineering domain, which is characterized by the following: the recombination plasmid is composed of -1349--743bp fragment of human BAFF gene upstream promotor sequence and alficetin acetylase(CAT); The usage and method are provided for preparing recombination plasmid; the recombination plasmid is exact and reliable; it's easy to activate and block-up controlling target dots of BAFF gene in transcription horizon.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Anti-CD40 Antibodies, Uses and Methods
ActiveUS20170240642A1Good effectSuppositories deliveryImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntigen
The present invention relates to antibodies (and fragments, variants, fusions and derivatives thereof) with multivalent binding specificity for CD40, which have a potency for dendritic cell activation which is higher than, or is equal to, the potency for B cell activation and wherein the antibody, antigen-binding fragment, or fusion, variant or derivative thereof has an affinity (KD) for CD40 of less than 1×10−10 M, which have utility in the treatment of diseases such as cancer. The invention also relates to pharmaceutical compositions, uses, methods and kits comprising such antibodies.
Owner:ALLIGATOR BIOSCI
Novel method for producing antibodies
PendingUS20210388060A1Promote antibody productionVertebrate antigen ingredientsMammal material medical ingredientsAntigenAntigen Binding Fragment
Methods for producing an antibody or an antigen-binding fragment thereof specifically binding to an antigen of interest, methods for inducing proliferation of PBMCs, B cell activation and differentiation, B cell maturation, and / or promoting class switch in an antibody-producing PBMC to produce IgG, compositions for the in vitro immunization and methods for identifying an antibody-enhancing factor for in vitro immunization.
Owner:TSINGHUA UNIV
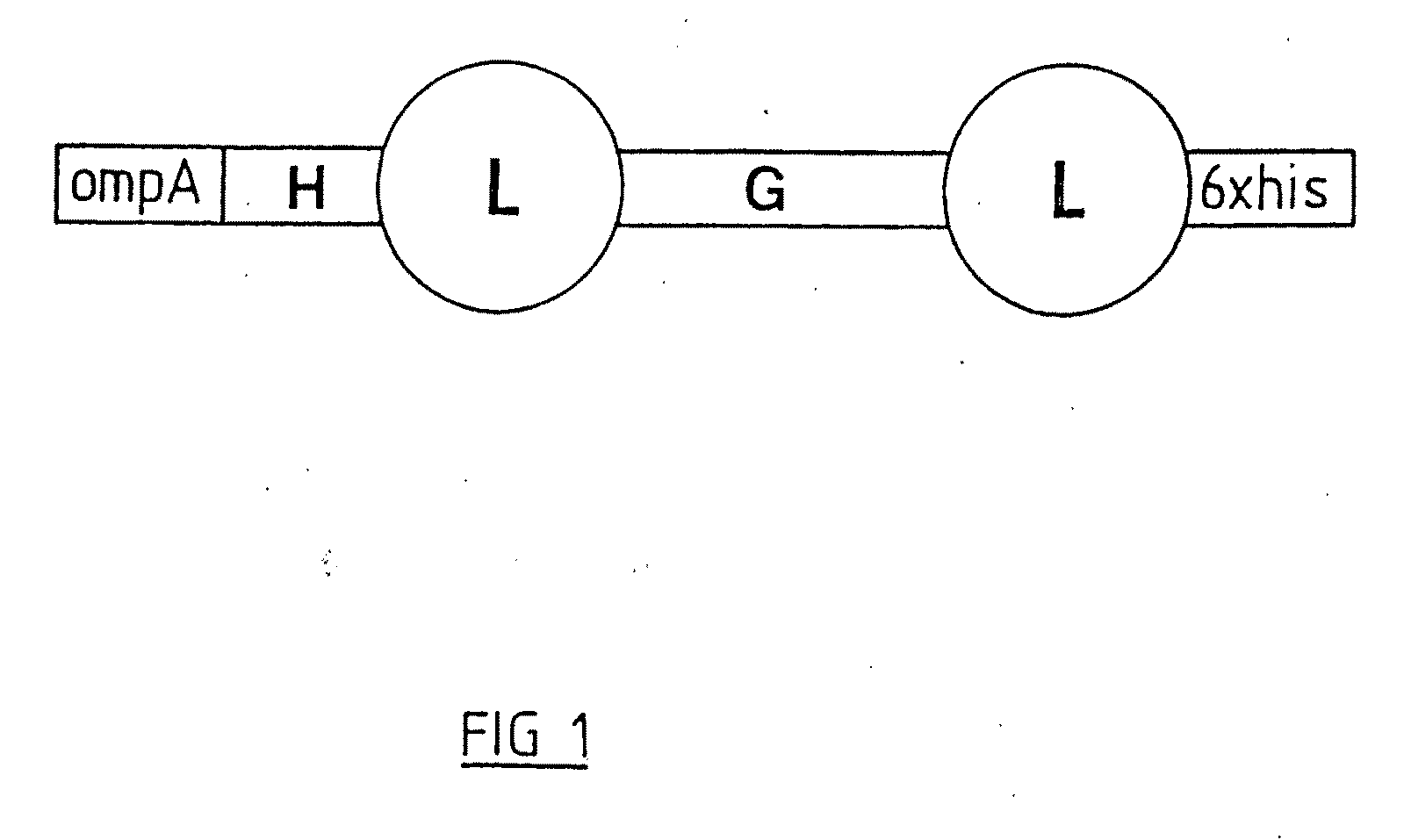
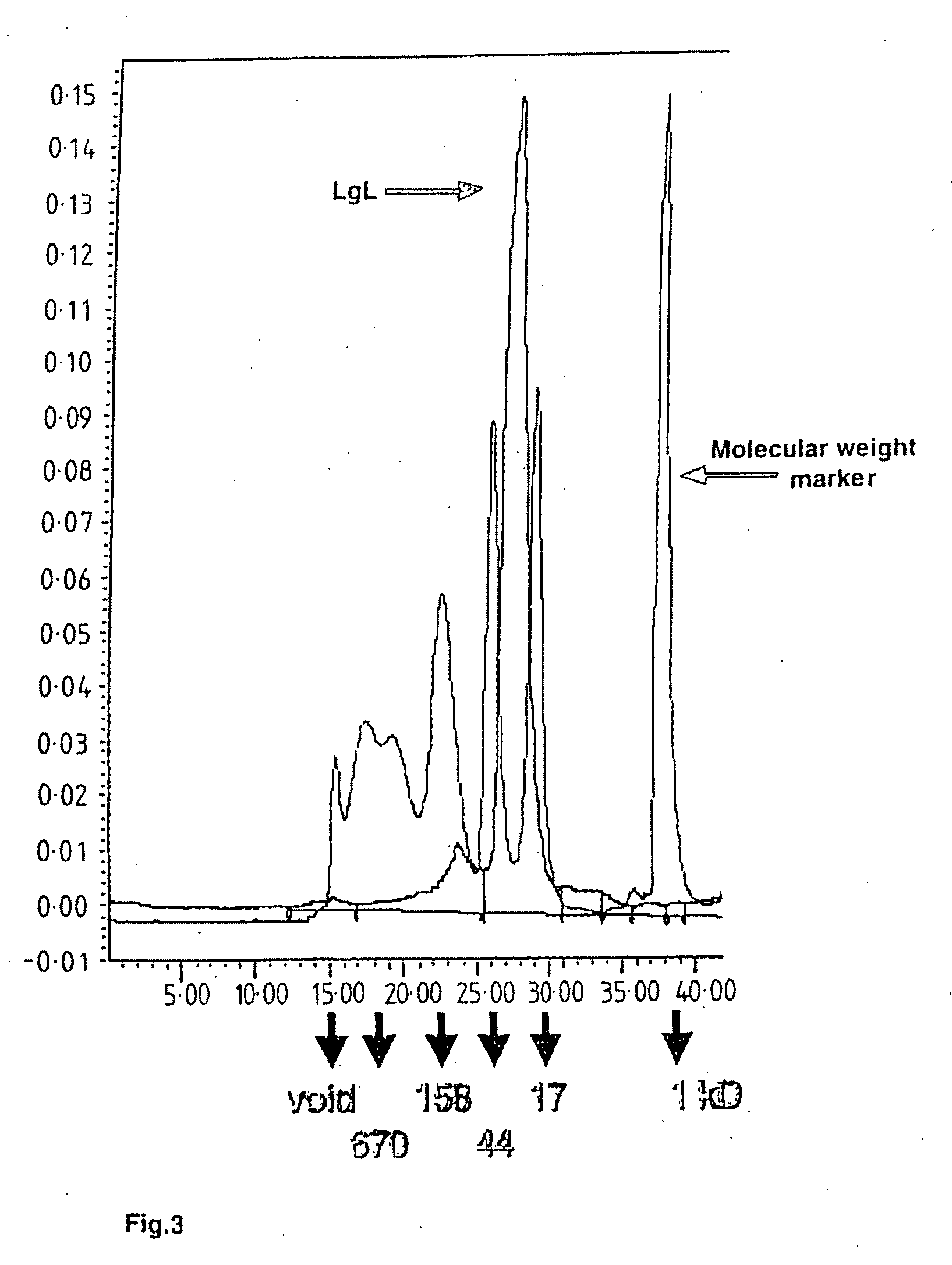
B Cell Activation and Polypeptides Having CD14 Activity
A novel protein purified from bovine colostral whey and isolated nucleotide sequences encoding the protein is identified. The isolated bovine protein is termed Bovine Lactation Associated Immunotropic (Bo-LAIT) protein. The human homologue of Bo-LAIT protein, Hu-LAIT protein, is also described. A method of activating B cells, and particularly of activating B cells in a mammal, such as a human, in need of such activation by administering LAIT protein is described. LAIT protein can be incorporated into infant formula. LAIT protein can be administered to an infant, as by feeding to the infant such formula. LAIT protein can be incorporated as part of a vaccination. LAIT protein can be administered to a patient having a T cell immune deficiency, for example, a particular T cell dysfunction in which gp39 (CD40L) is under expressed on or totally absent from the cell surface of patient T cells. Preparation of medicaments including LAIT protein for activating B cells in a mammal in need of such activation is described. Natural or recombinant LAIT protein can be used.
Owner:THE ARTHRITIS & AUTOIMMUNITY RES CENT FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com