Mutant encoding gene of human 4IgB7-H3 and application thereof in regulating immunity
A 4igb7-h3, encoding gene technology, applied in the direction of anti-animal/human immunoglobulin, application, genetic engineering, etc., can solve the problem that there is no 4IgB7-H3 mutation encoding gene reported, and achieve the effect of enhanced inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
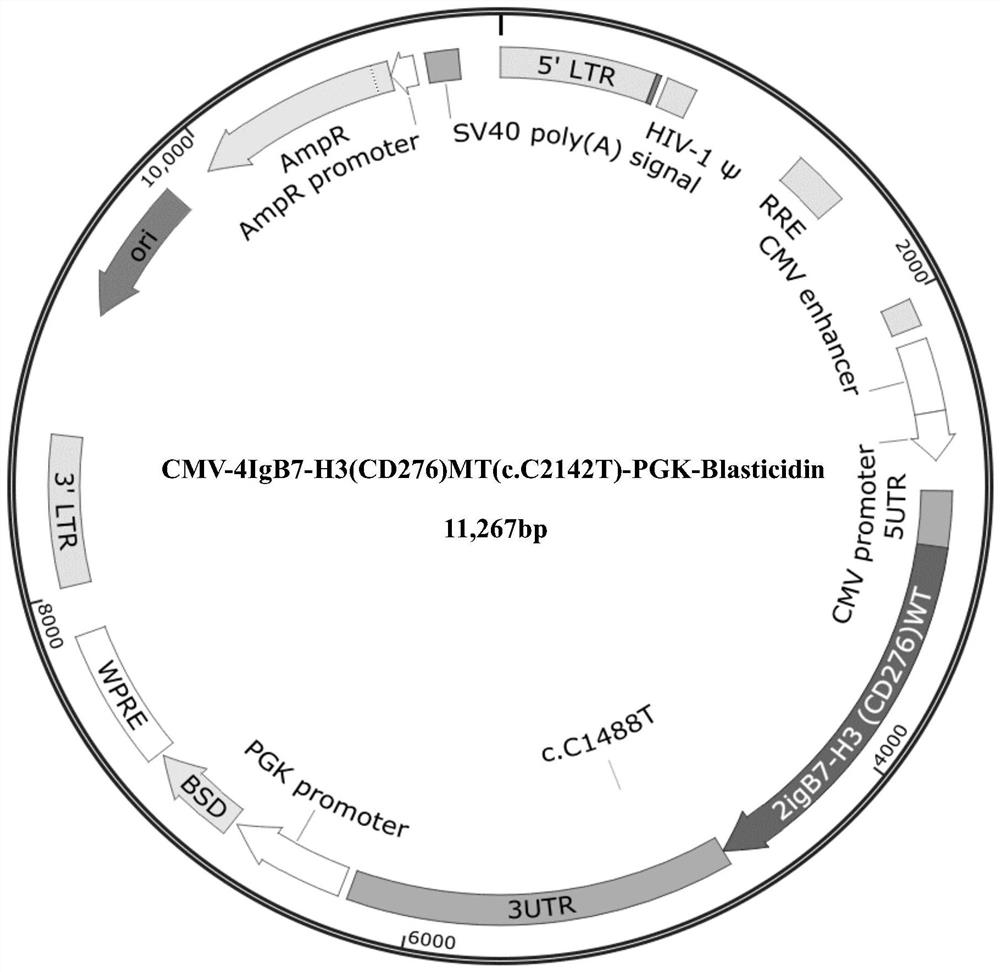
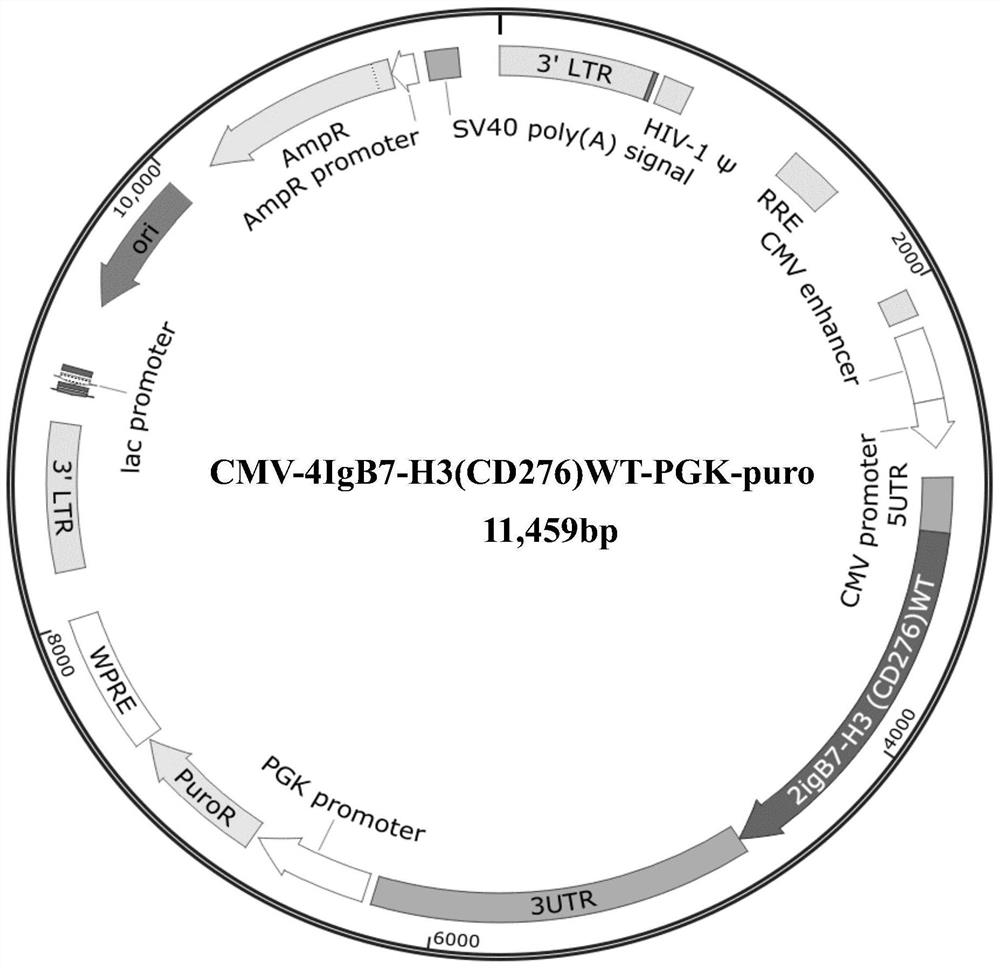
[0045] Example 1 Preparation of plasmid containing 4IgB7-H3 wild-type coding gene and 4IgB7-H3 mutant coding gene
[0046] 1. Materials and methods
[0047] After PCR, the artificially synthesized wild-type 4IgB7-H3 coding gene (SEQ ID NO: 2, NCBI Reference Sequence: NM_001024736.1) and 4IgB7-H3 mutant coding gene (SEQ ID NO: 1) were constructed into PGMLV-PA7 and PGMLV-PA6, and Blasticidin and puro were used as screening markers, respectively. The vector was transformed into competent cells by conventional techniques in the field, and the competent cells were plated and placed upside down in a 37°C constant temperature incubator (Shanghai Yiheng Scientific Instrument Co., Ltd.) for overnight culture. On the second day, single clones were selected, and two of each carrier clone were selected and sent to the sequencing company for sequencing identification. After comparison, the sequence of the insert fragment in the recombinant clone was completely consistent with the sequenc...
Embodiment 2
[0072] The packaging of embodiment 2 lentivirus and the mensuration of virus titer
[0073]Cell line: HEK 293T, the packaging cell of lentivirus, is an anchorage-dependent epithelioid cell, and the growth medium is DMEM (containing 10% FBS). Adherent cells grow and proliferate in culture to form a monolayer of cells.
[0074] Strain: E. coli strain DH5α. For amplification of lentiviral vectors and helper packaging vector plasmids.
[0075] Lentiviral packaging system: The successfully constructed lentiviral recombinant plasmids and packaging plasmids were extracted using Qiagen's plasmid extraction kit. The obtained plasmid DNA was dissolved in sterile TE, and its concentration and purity were measured by ultraviolet light absorption method to ensure that the A260 / A280 of the extracted plasmid DNA was between 1.8 and 2.0.
[0076] 1. Lentiviral packaging
[0077] 1) HEK 293T cell division: The day before transfection, subculture the grown cells into a 10cm culture dish at ...
Embodiment 3
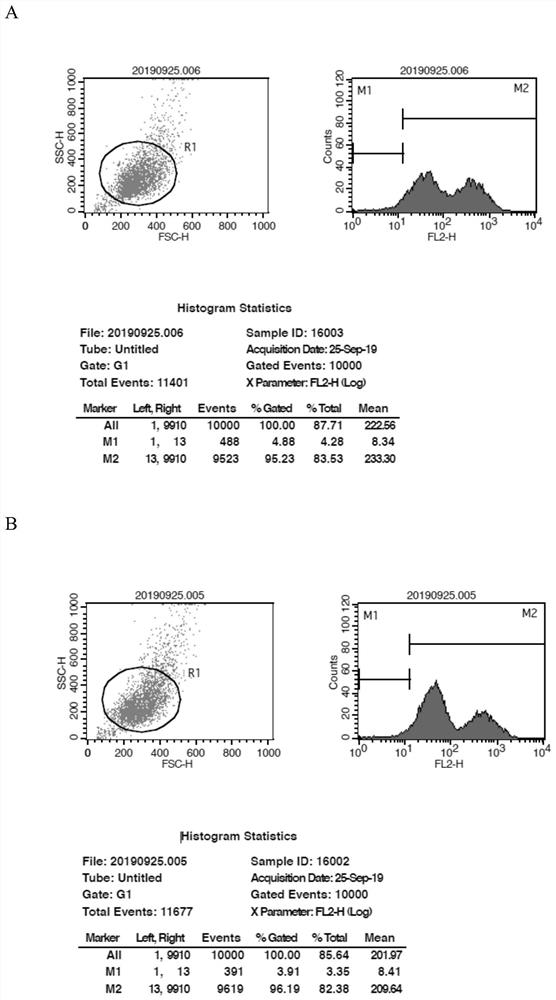
[0106] Example 3 Comparison of Mutant and Wild-type HEK293T Cell Effects in Vitro
[0107] 1. Lentivirus infection of target cells
[0108] On the first day, target cells HEK293T (purchased from the Shanghai Chinese Academy of Sciences Cell Bank) were inoculated in a culture dish at an appropriate ratio.
[0109] On the next day, before infection, the virus stock solution was taken out from the -80°C refrigerator and melted in an ice bath, and the virus stock solution was diluted with a fresh medium containing 5 μg / ml Polybrene according to an appropriate MOI value, and the original culture medium of the control group and the treatment group was aspirated. Base, the dilution containing the lentivirus negative control (NC-) was added to the cells of the control group, and the dilution containing the lentivirus solution was added to the cells of the treatment group.
[0110] On the third day, the culture medium was replaced, and the culture medium containing the lentivirus was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com