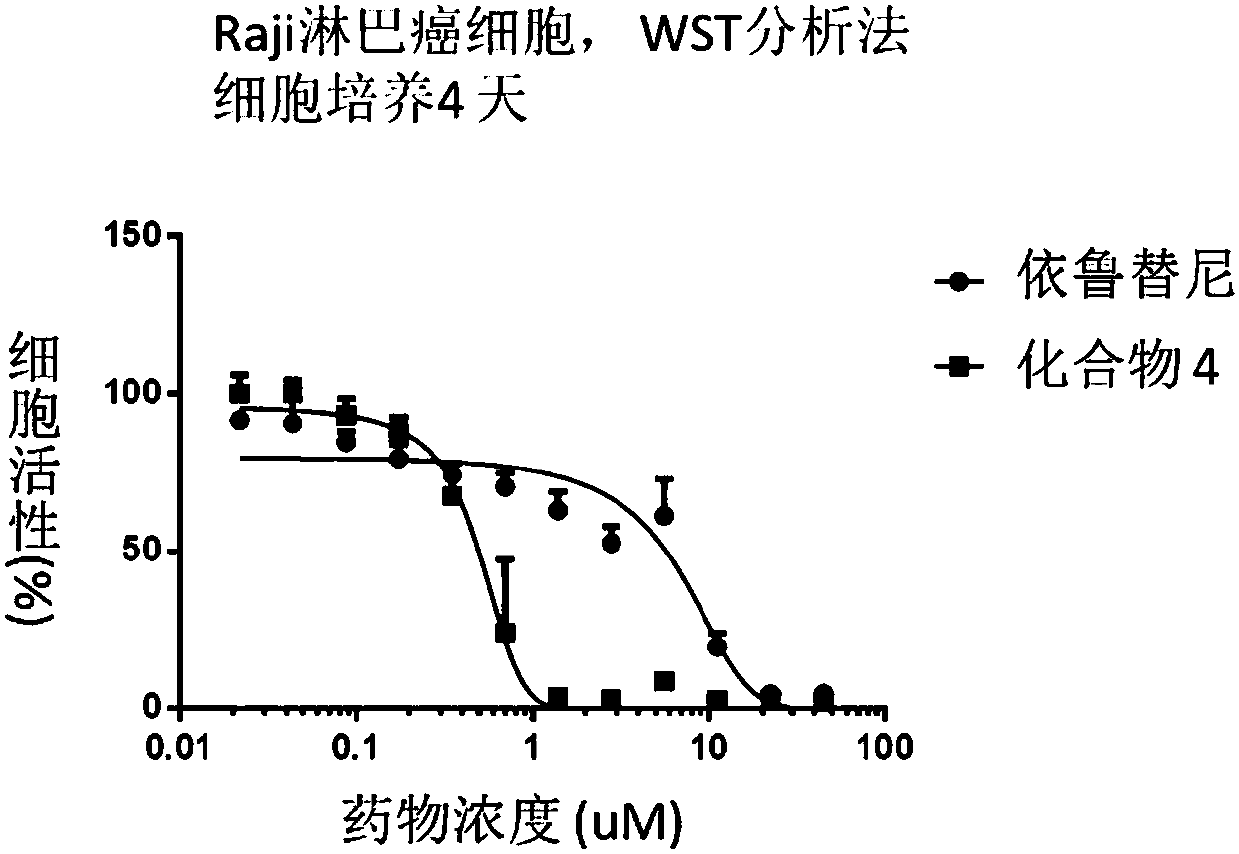
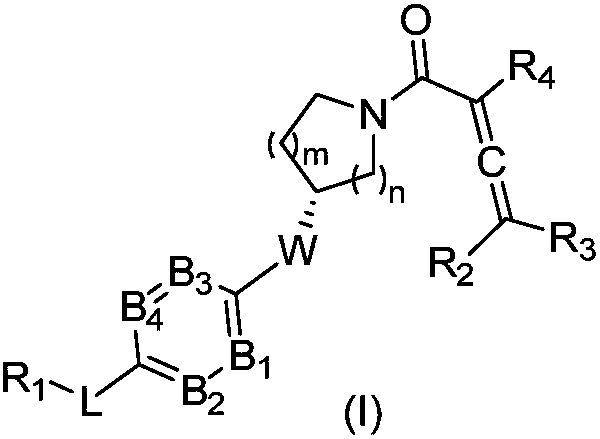
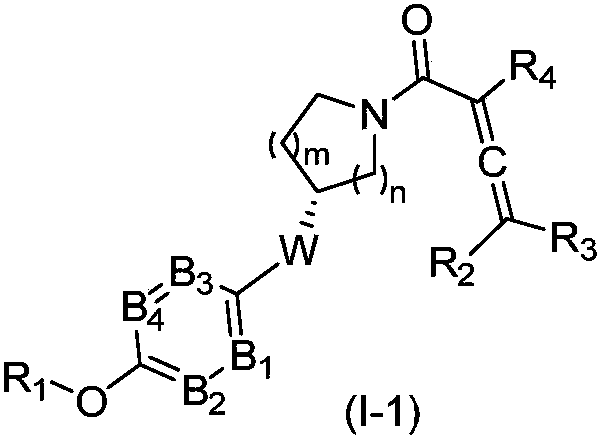
Compounds containing conjugated allene structure, and pharmaceutical compositions and application thereof
A compound, C1-C4 technology, applied in the prevention or treatment of diseases related to abnormal B cell activity, in the field of B cell activation inhibitors, can solve the problems of uncertain properties, unknown biological activities and pharmacological properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0159] The synthesis of preparation embodiment 1 boric acid, borate:
[0160] Method 1: Synthesis of N-(2-pyridyl)4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolyl)-benzamide (C007)
[0161]
[0162] Step 1, synthesis of p-bromobenzoyl chloride: in a 250mL glass flask, add p-bromobenzoic acid (3g, 15mmol), N,N-dimethylformamide (DMF, 0.4mL) and dichloromethane (CH 2 Cl 2 , 50mL). The reaction solution was cooled to zero with an ice-water bath, and thionyl chloride (SOCl 2 , 1.32 mL, 18 mmol). The reaction was warmed to room temperature and stirred overnight. Evaporated to dryness by rotary evaporation, the obtained crude product was purified by co-evaporation with toluene (30 mL×2), and a colorless liquid was obtained which was directly used in the next reaction.
[0163] Step 2, synthesis of N-(2-pyridyl)-p-bromobenzamide (C004): put all the colorless liquid obtained in the previous step into a 250 mL glass flask, and dissolve it in dry tetrahydrofuran (THF, 20 mL). This so...
preparation Embodiment 2
[0183] Preparation Example 2 Synthesis of the key intermediate N-Cbz-(S)-3-(1-bromo,8-aminoimidazo[1,5-a]pyrazinyl)-substituted-2-pyrrolidine (S8)
[0184]
[0185] 2-(3-Chloropyrazin-2-yl)methanamine(S4)
[0186] Step 1, synthesis of 2-(3-chloropyrazinyl) methyl bromide (S2): 2-chloro-3-methylpyrazine (S1, 7.72g, 60mmol) and N-bromosuccinimide ( NBS, 14.87g, 84mmol) dissolved in carbon tetrachloride (CCl 4 , 100mL). One-time addition of benzoyl peroxide (Bz 2 o 2 , 1.45g, 6mmol), the reaction solution was refluxed overnight. Then, the reaction solution was cooled to room temperature, the solid was removed by filtration, and the solid was washed with carbon tetrachloride to obtain CCl 4 solution. The carbon tetrachloride solutions were combined and concentrated to obtain 13.27 g of a mixture containing S2, which was directly used in the next reaction without purification.
[0187]Step 2, Synthesis of 2-(3-chloropyrazinyl)methyl-substituted-2-isoindole-1,3-dione (S3):...
Embodiment 1
[0200] Embodiment 1: synthetic compound (S)-N-(pyridin-2-yl) para-position (3-(1-(2,3-butenoyl) pyrrolidin-2-yl)-8-aminoimidazole[ 1,5-a]pyrazin-1-yl)benzamide (1)
[0201]
[0202] Step 1: Synthesis of (S)-2-((1-(4-(pyridin-2-ylcarbamoyl)phenyl)-8-amino)imidazo[1,5-a]pyrazin-2-yl ) benzyl pyrrolidin-1-ylcarboxylate (C018)
[0203] In a 100mL glass bottle, add 4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolyl)-benzoic acid 2-pyridylamineamide (213mg, 0.66 mmol), S8 (137 mg, 0.33 mmol) and 1,2-dimethoxyethane (4 mL). Aqueous sodium carbonate solution (2M, 2 mL) was added to the above solution, and the solution was deoxygenated. Then, [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex (24 mg) was added to the reaction flask and oxygen was removed again. The reaction solution was refluxed overnight, cooled to room temperature, and the aqueous phase was extracted with ethyl acetate. The organic phases were combined, washed with saturated brine, dried,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com