B Cell Activation and Polypeptides Having CD14 Activity
a technology of cd14 activity and polypeptides, which is applied in the field of b cell activation and polypeptides having cd14 activity, can solve the problems of limited susceptibility of b cells to these cytokines and the inability to respond to subsequent progression signals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
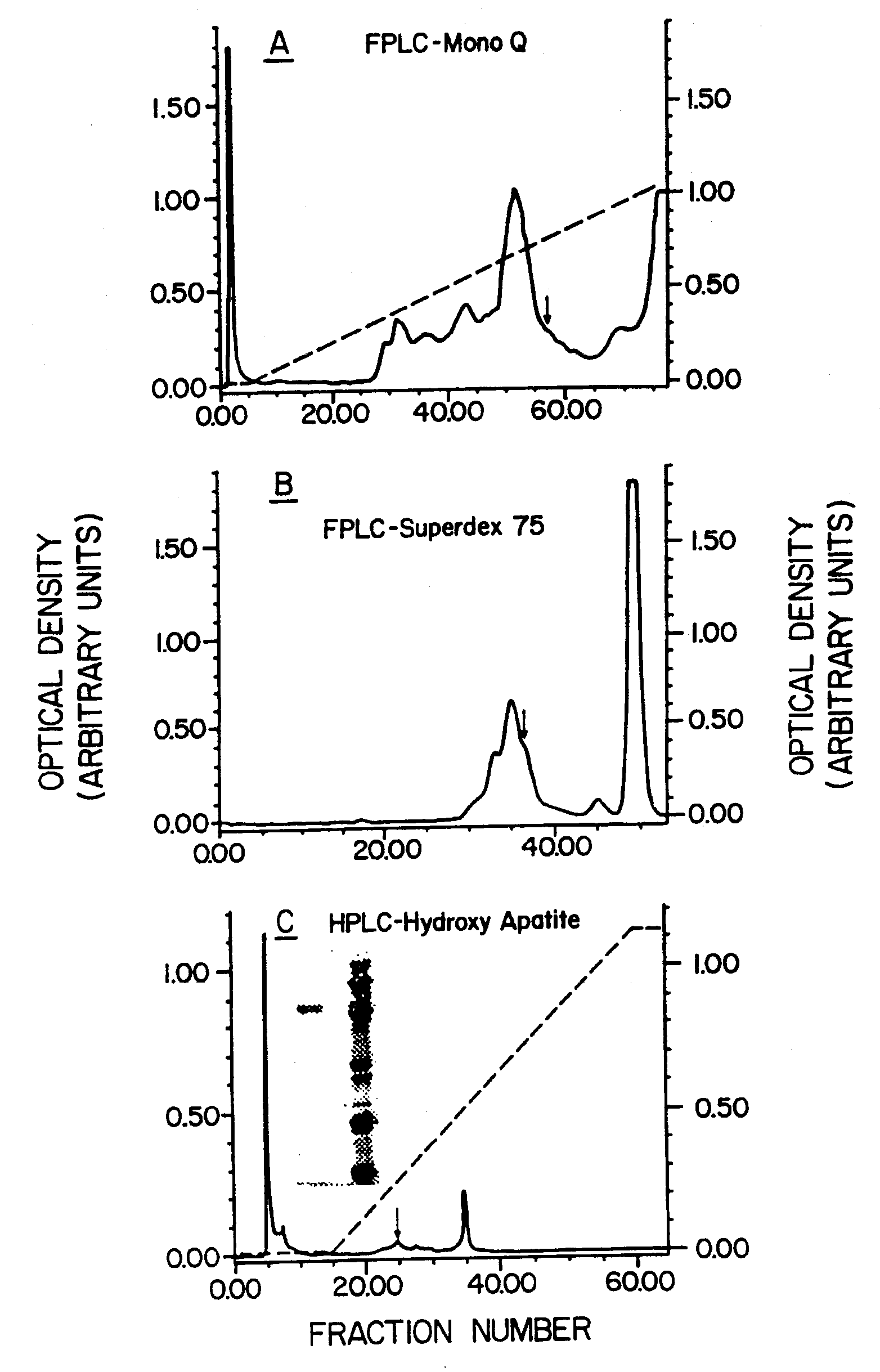
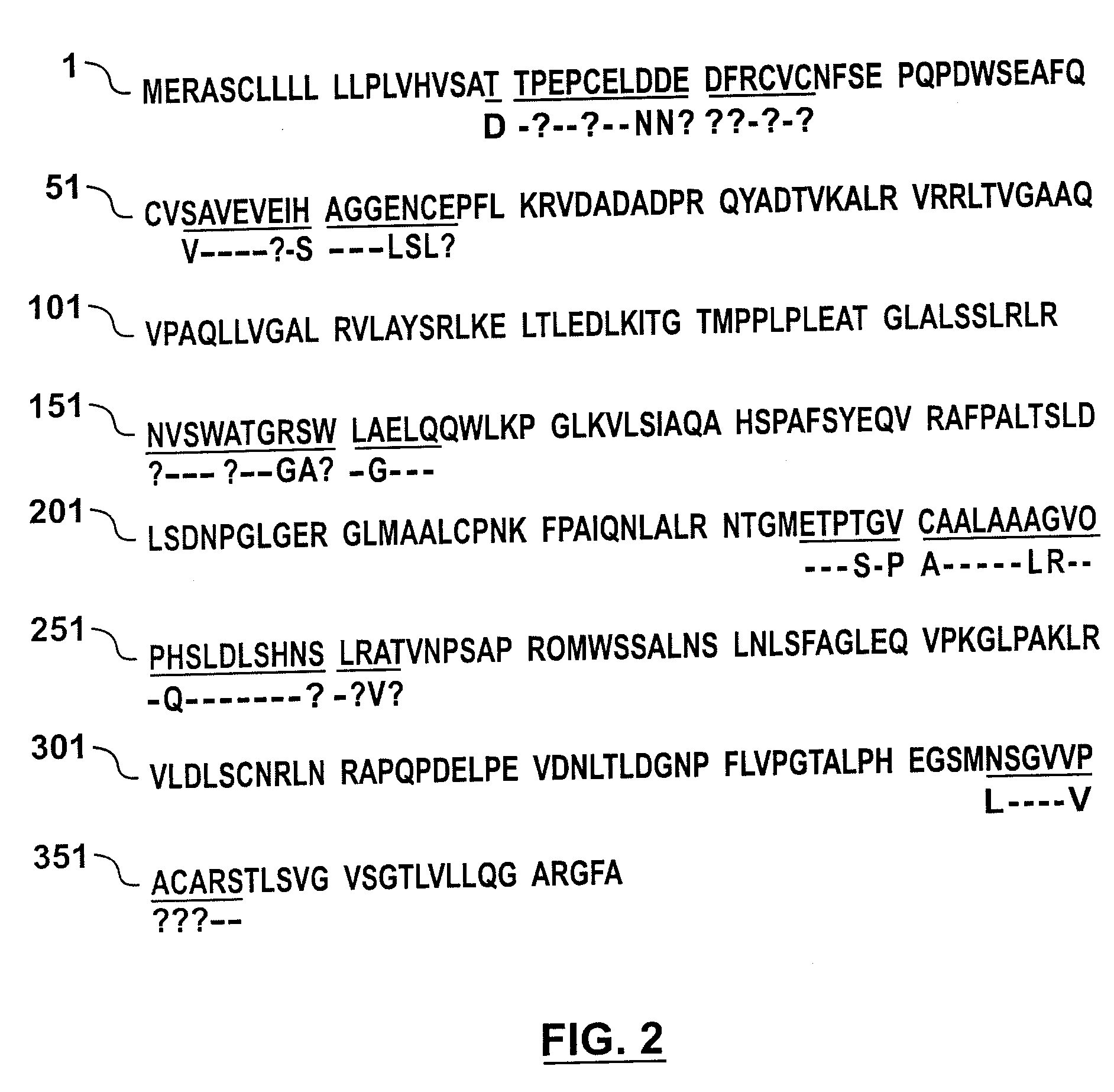
[0060] The experiments described below demonstrate purification of native bovine LAIT protein (nBo-LAIT), also referred to herein as bovine CD14, from colostral whey. Amino acid sequence analysis of purified nBo-LAIT is shown, and homology with human CD14 is demonstrated. A method for the purification of human CD14 from is shown. A method for the purification of mouse CD14 from hybridoma supernatant is shown.
[0061] In vitro B cell stimulation assays are described for affinity purified colostral Bo-LAIT, human colostral CD14, human CD14 derived from urine, and mouse CD14 derived from a hybridoma supernatant. High buoyant density resting splenic B cells derived from mouse are shown to enter and progress through cell cycle, in response to LAIT protein from the three species, and to differentiate into high rate immunoglobulin secreting cells in response to exposure to LAIT-protein from bovine. These activation events occur in defined serum free medium, and it is also shown that the pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com