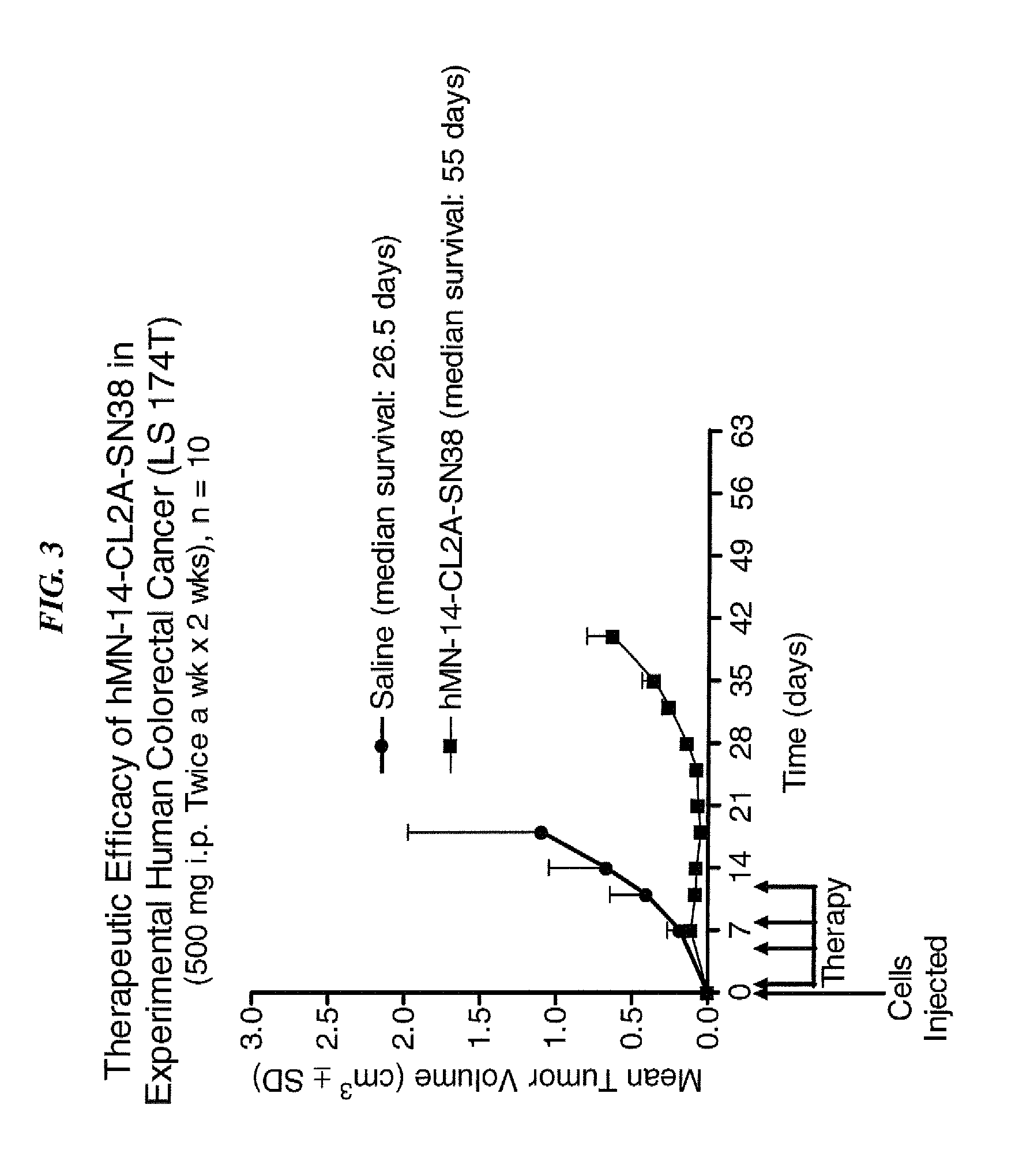
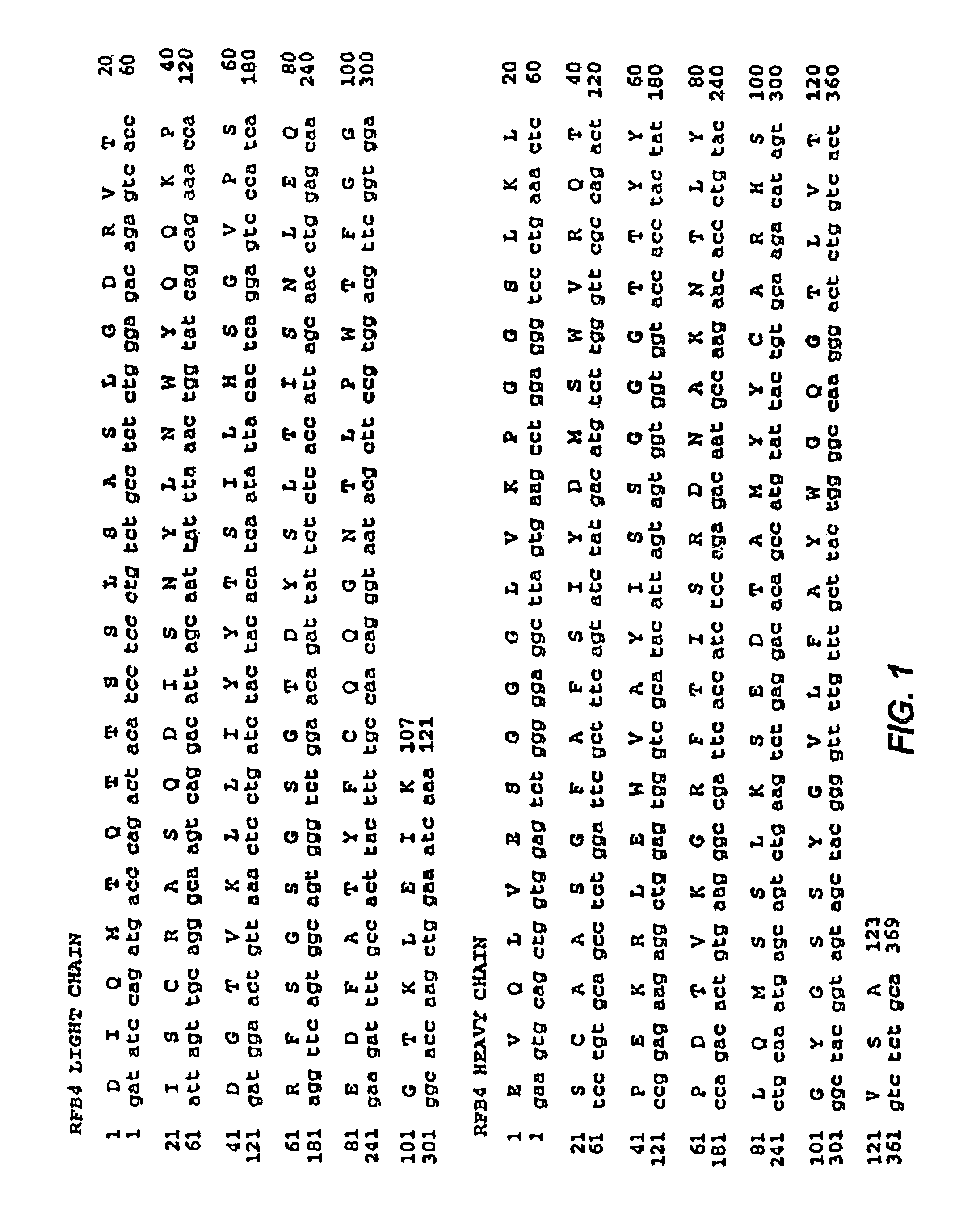
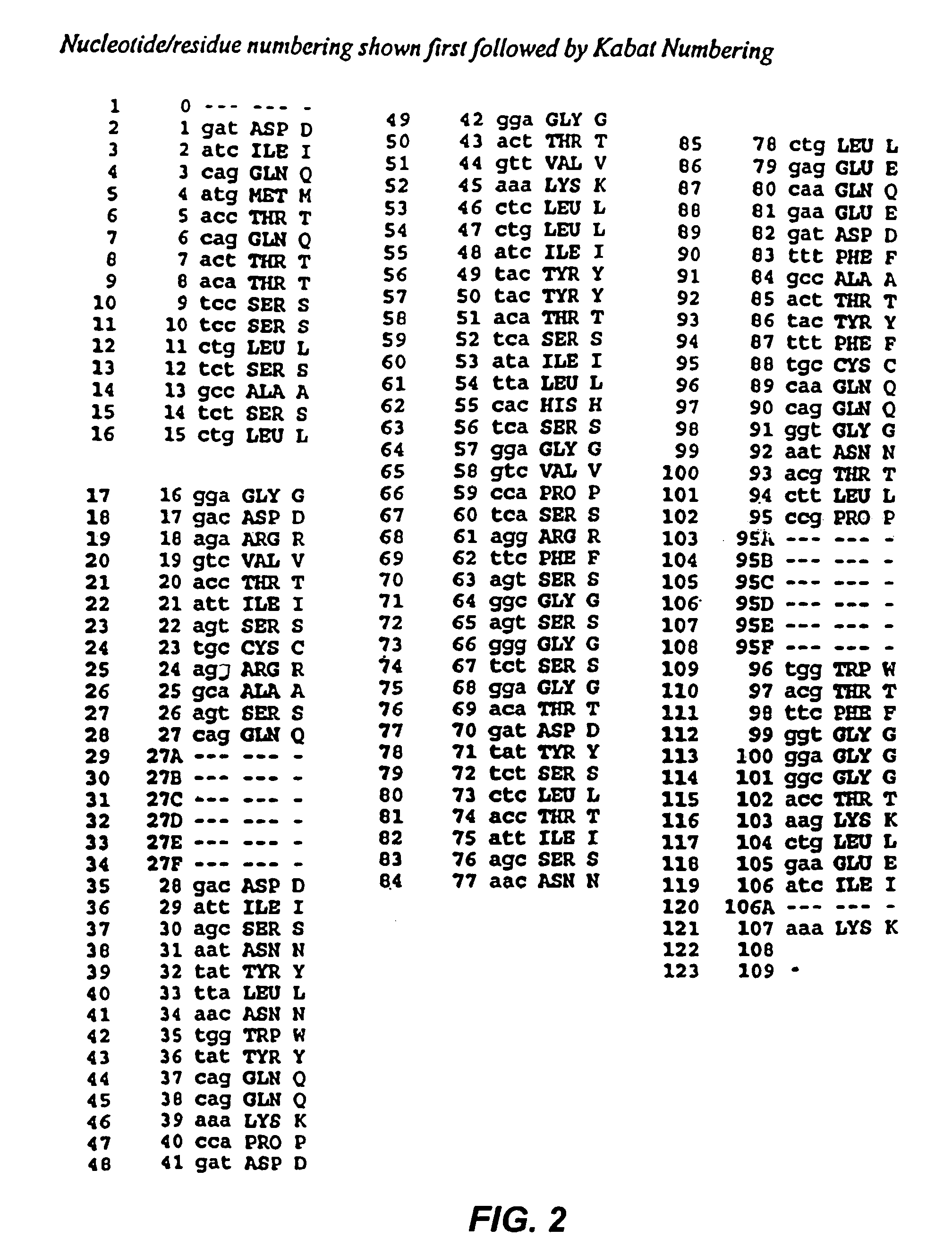
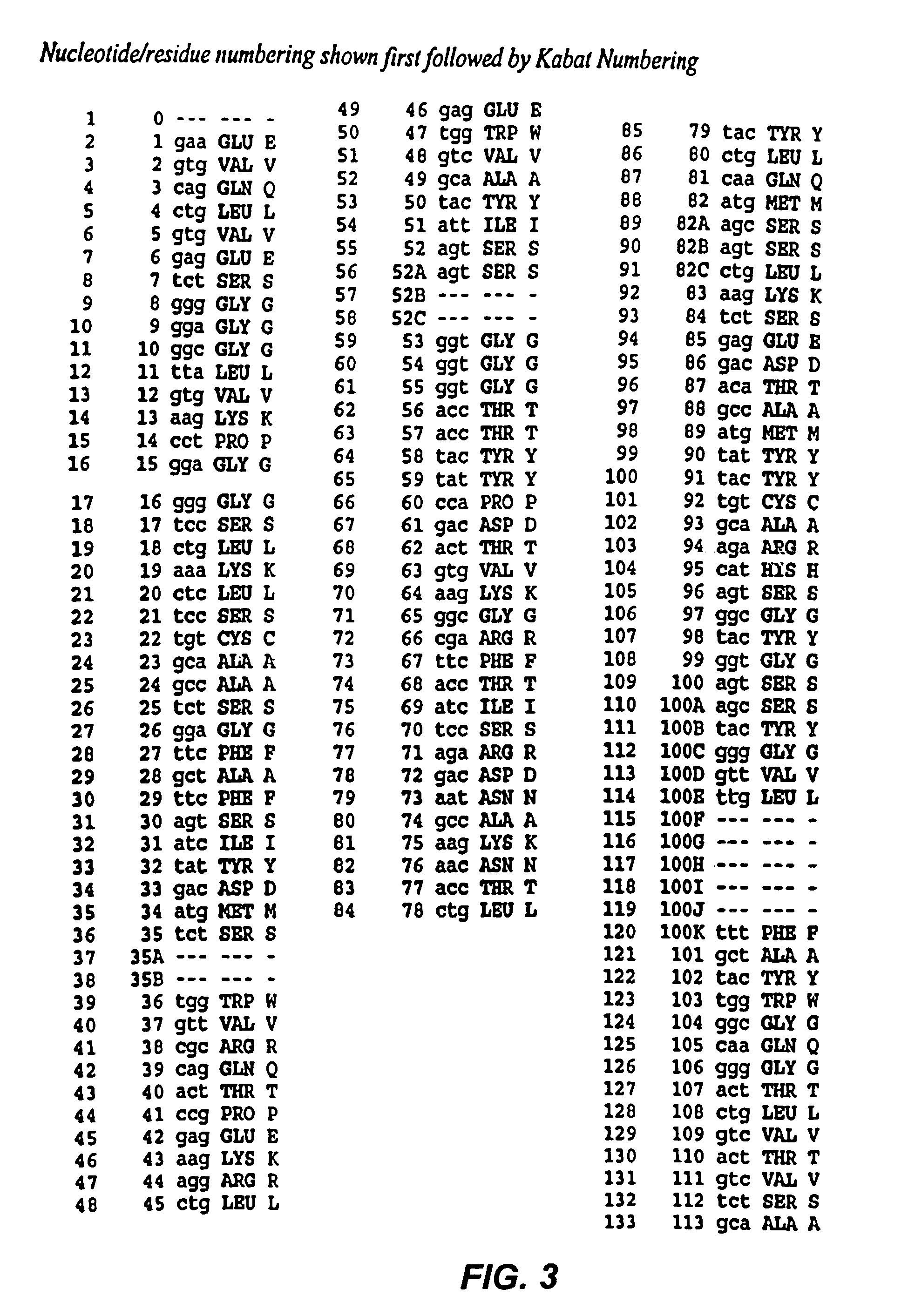
Patents
Literature
147 results about "CD22" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CD22, or cluster of differentiation-22, is a molecule belonging to the SIGLEC family of lectins. It is found on the surface of mature B cells and to a lesser extent on some immature B cells. Generally speaking, CD22 is a regulatory molecule that prevents the overactivation of the immune system and the development of autoimmune diseases.
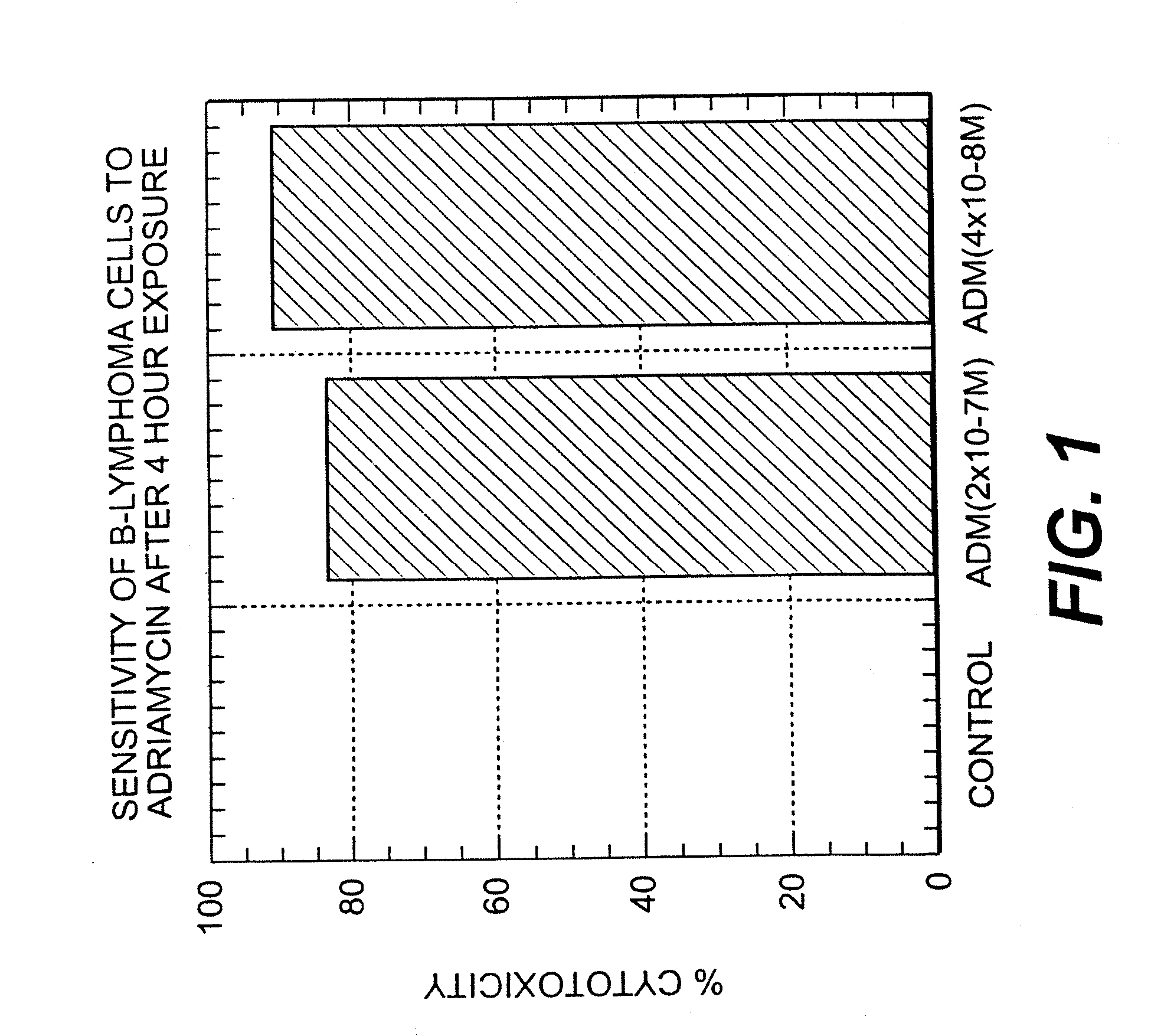
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20020012665A1Avoiding and decreasing and resistanceOrganic active ingredientsIn-vivo radioactive preparationsFactor iiBiological activation
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN(R).
Owner:BIOGEN INC
Immunoregulatory antibodies and uses thereof
InactiveUS20030103971A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
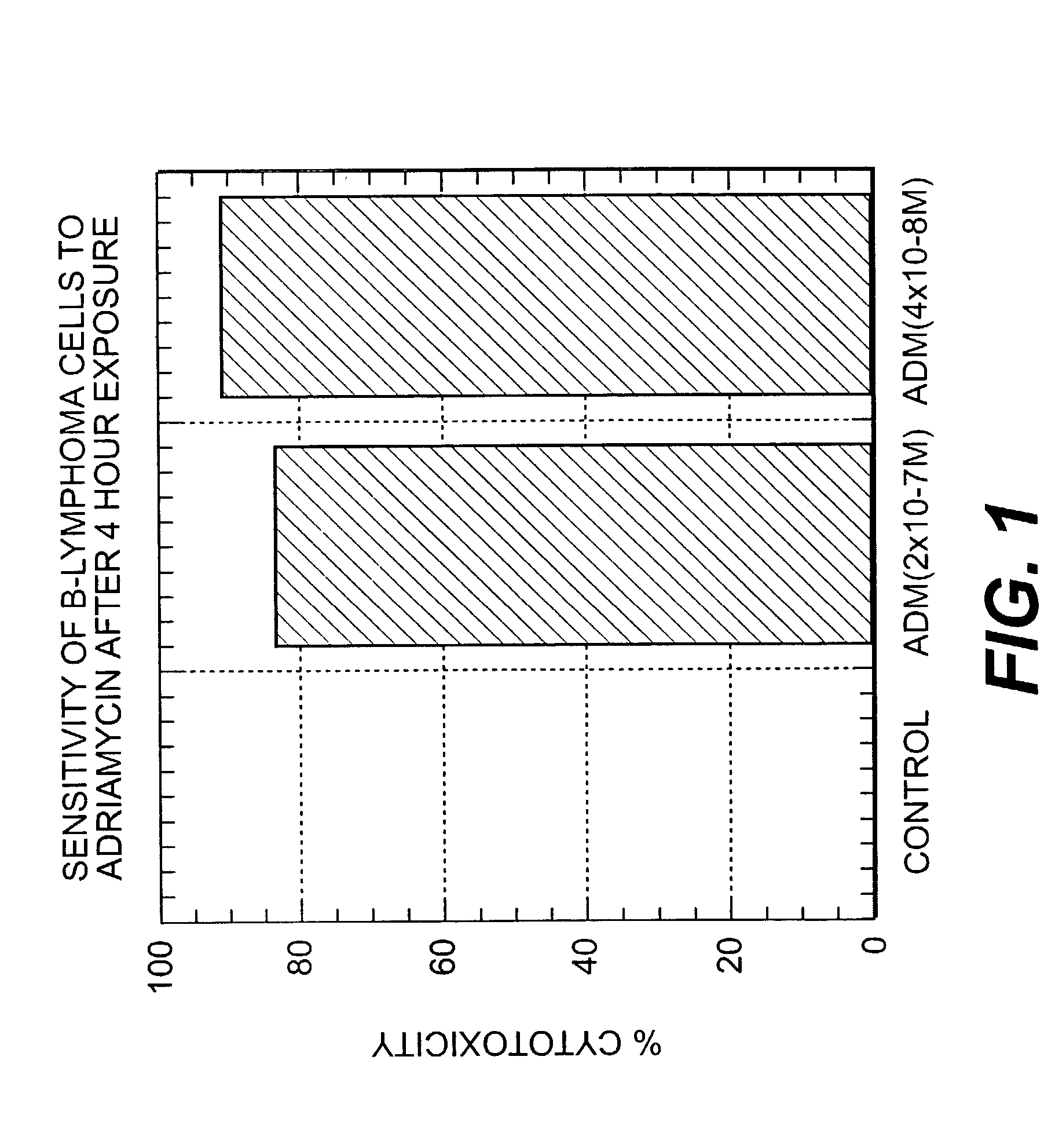
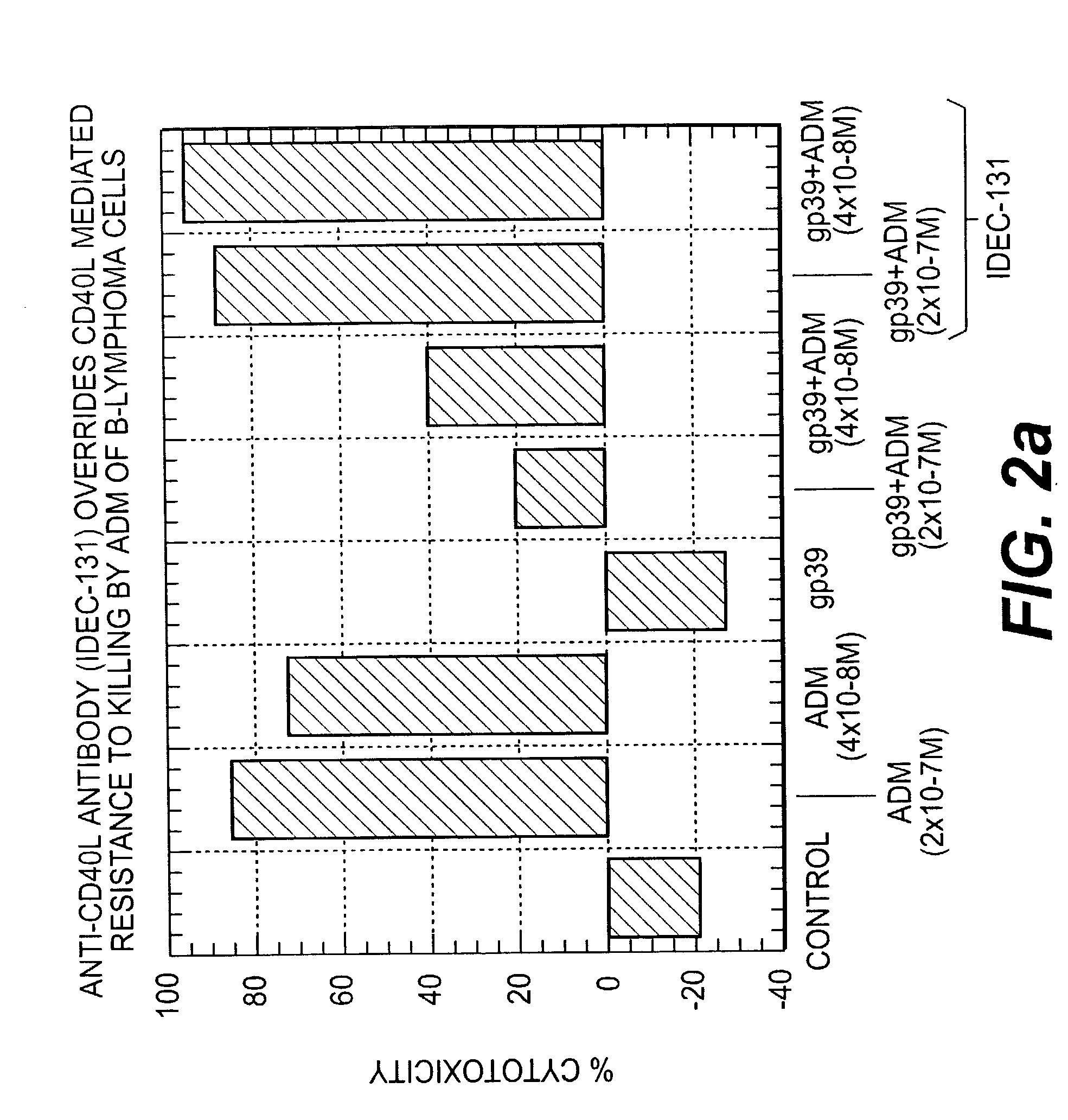
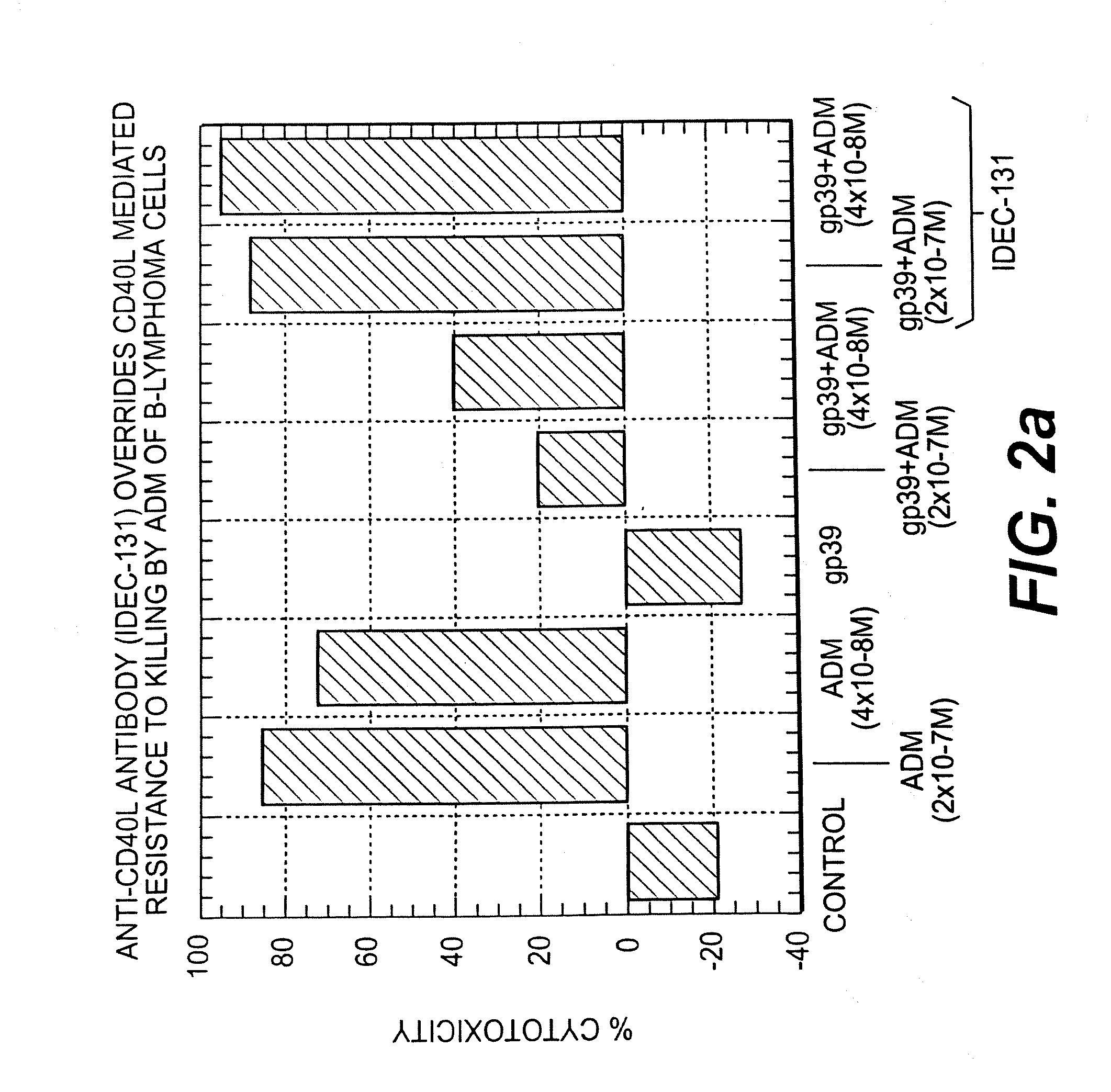
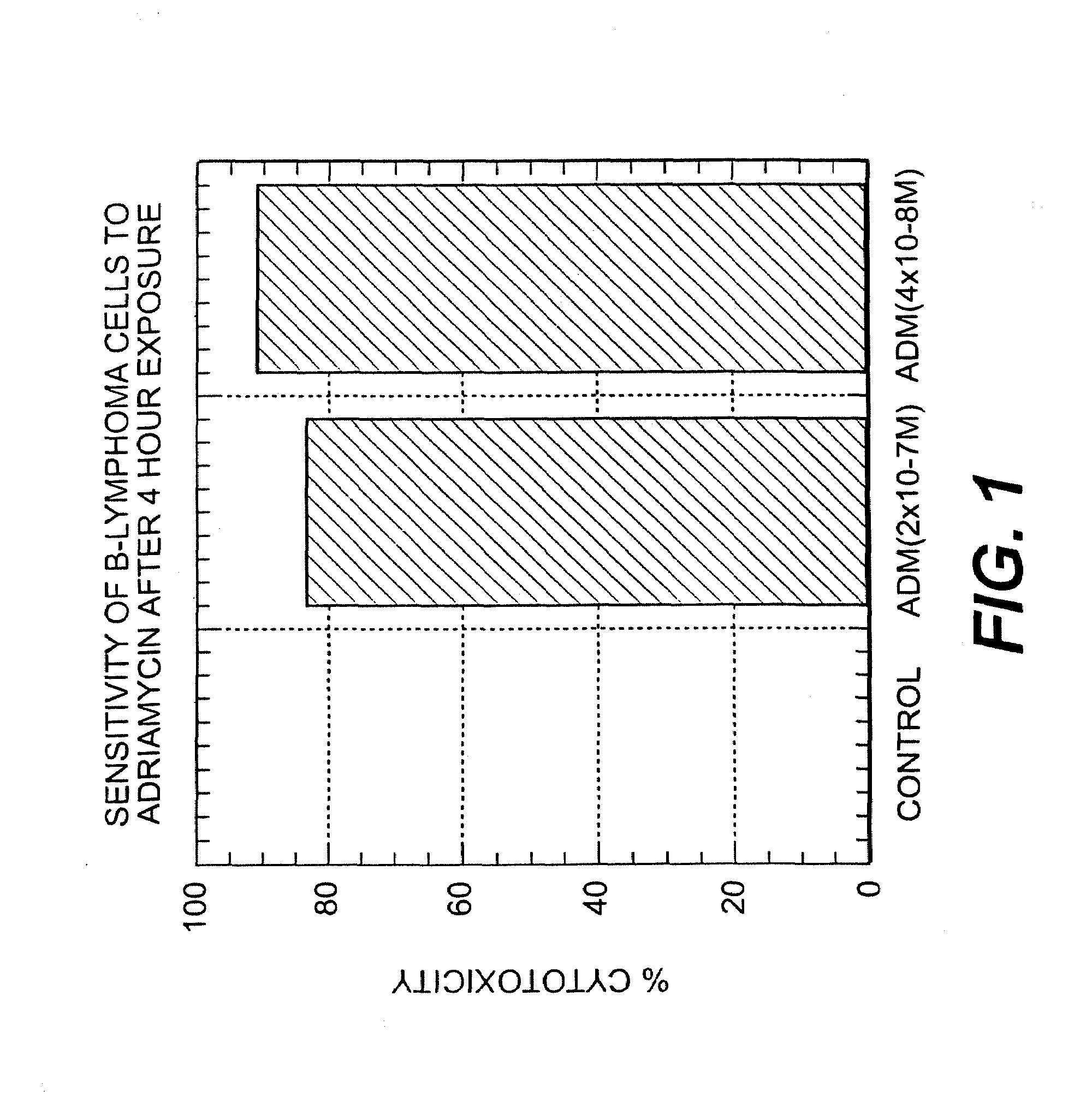
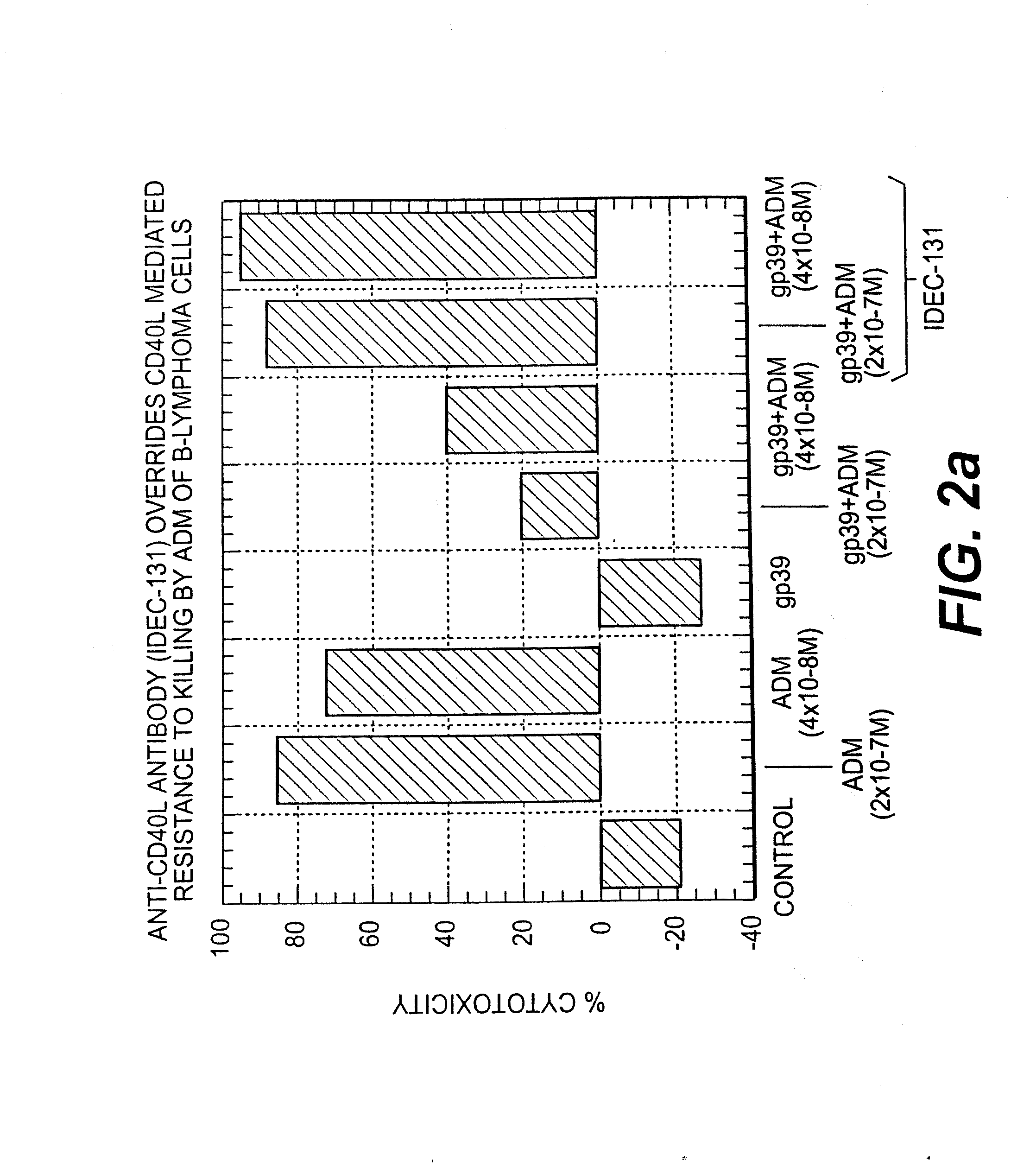
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS6896885B2Avoiding and decreasing and resistanceIncrease ratingsOrganic active ingredientsIn-vivo radioactive preparationsBiological activationHematologic malignancy
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies.The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN® (rituximab).
Owner:BIOGEN INC
Treatment of B-cell associated diseases
InactiveUS6846476B2Enhanced killing and depletionReduce the possibilityRadioactive preparation carriersAntibody ingredientsDiseaseAutoimmune responses
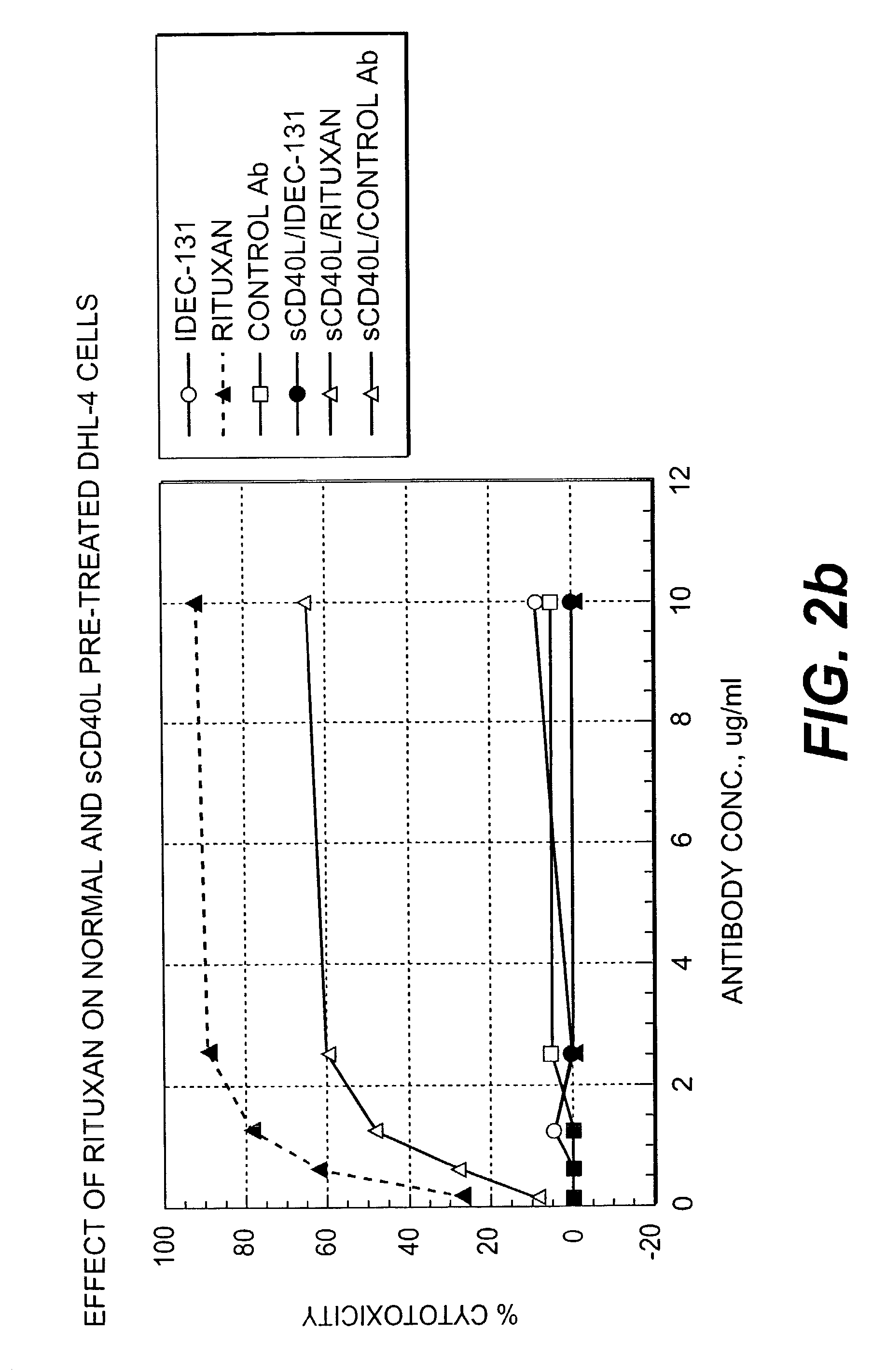
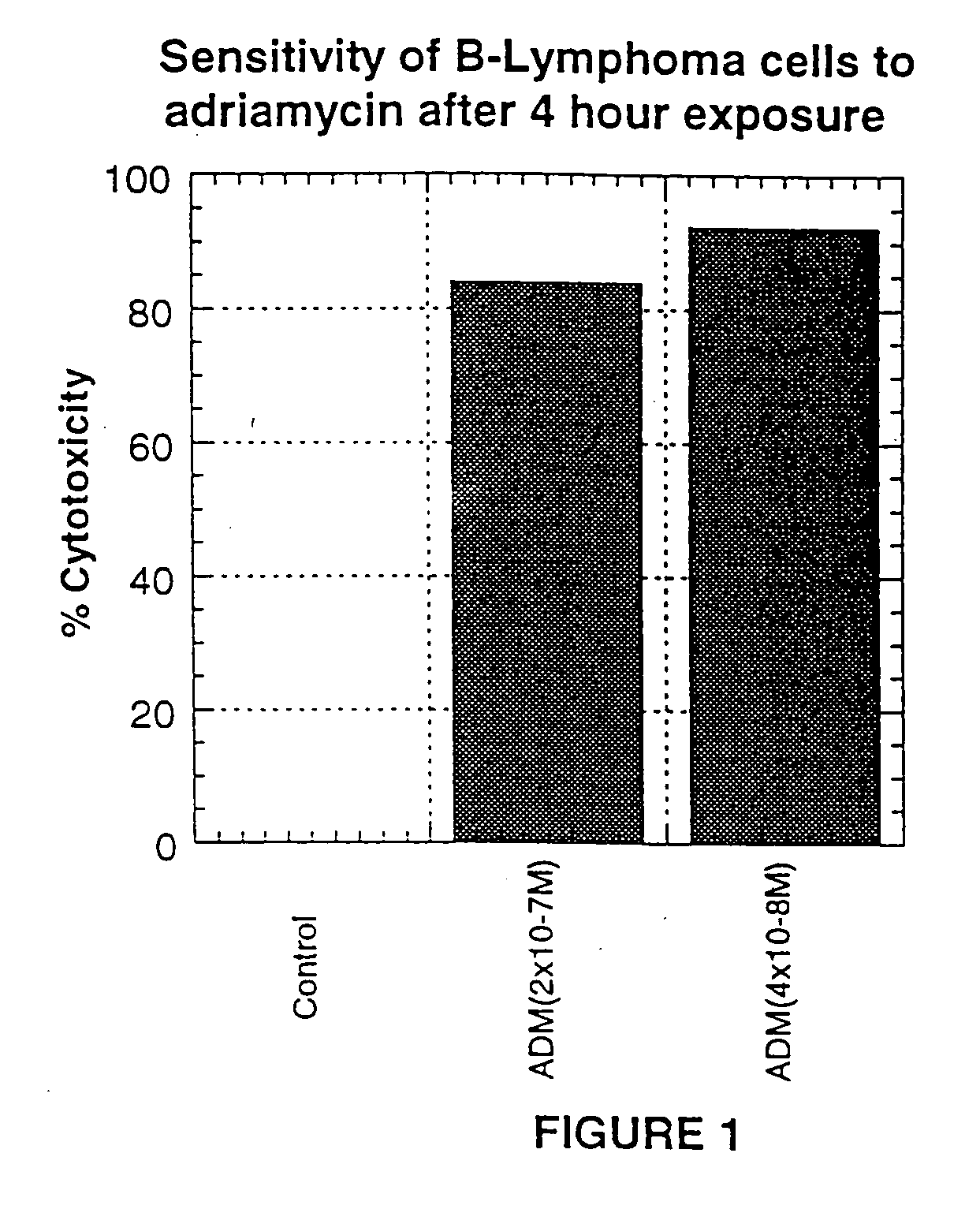
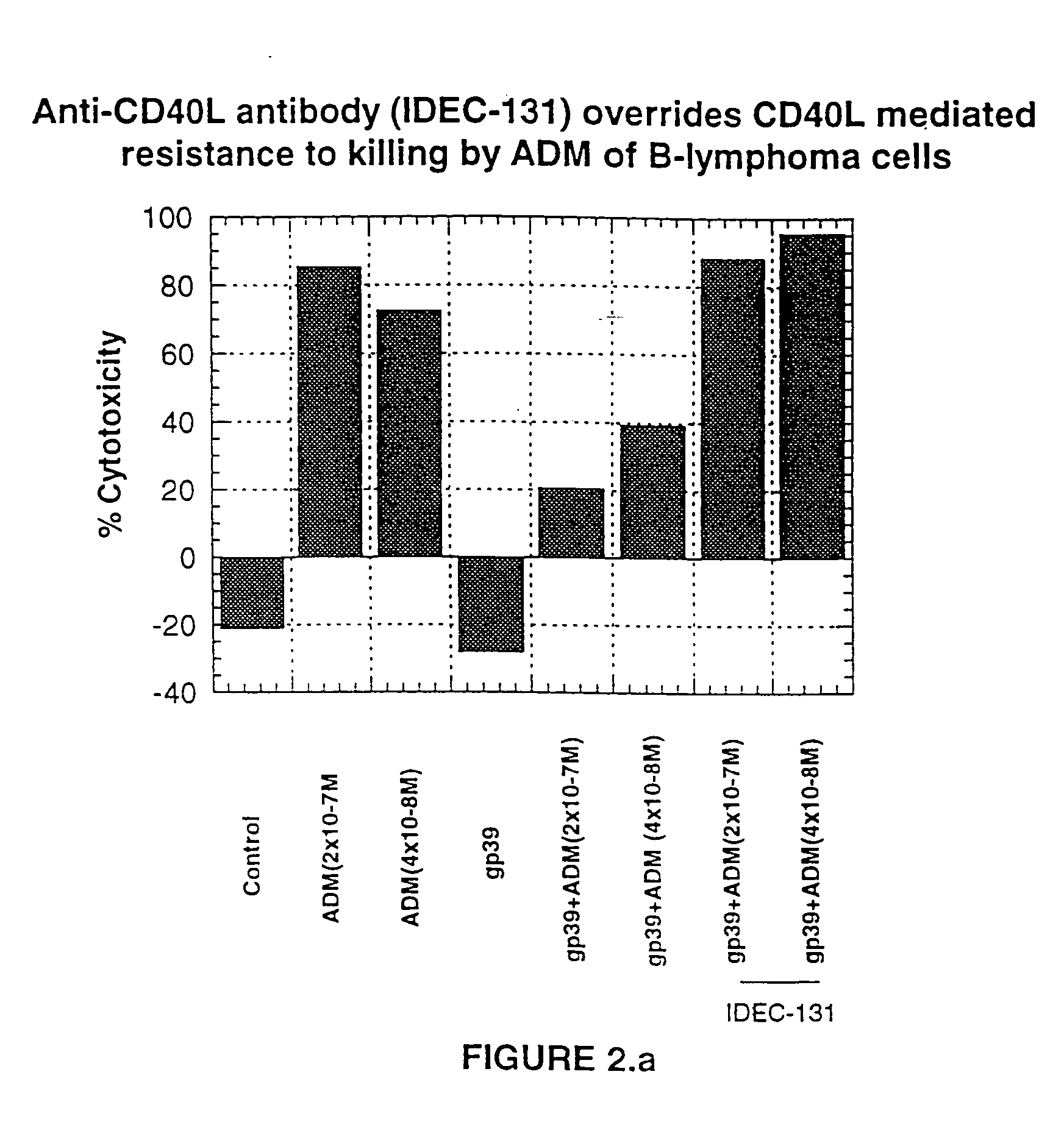
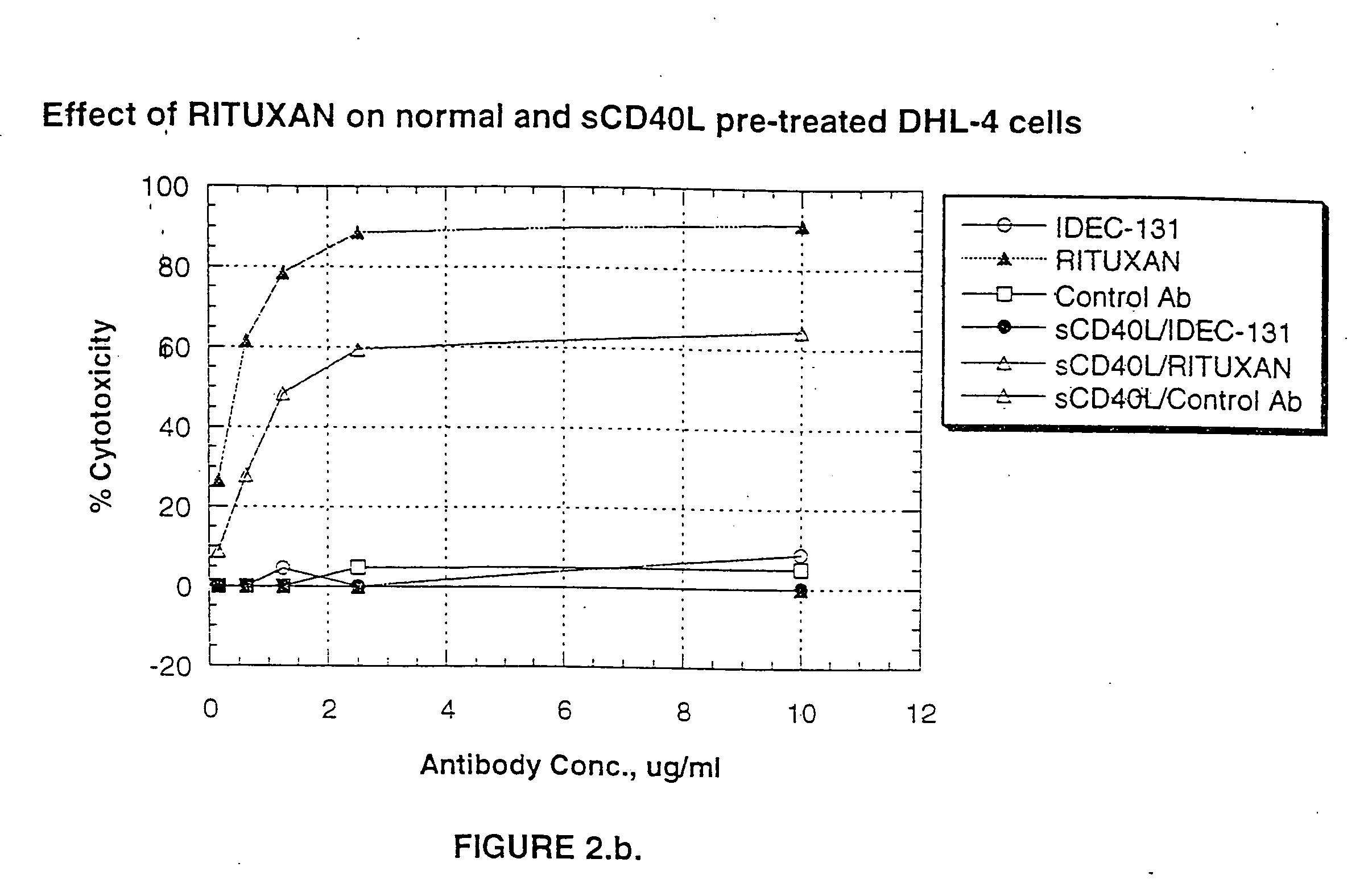
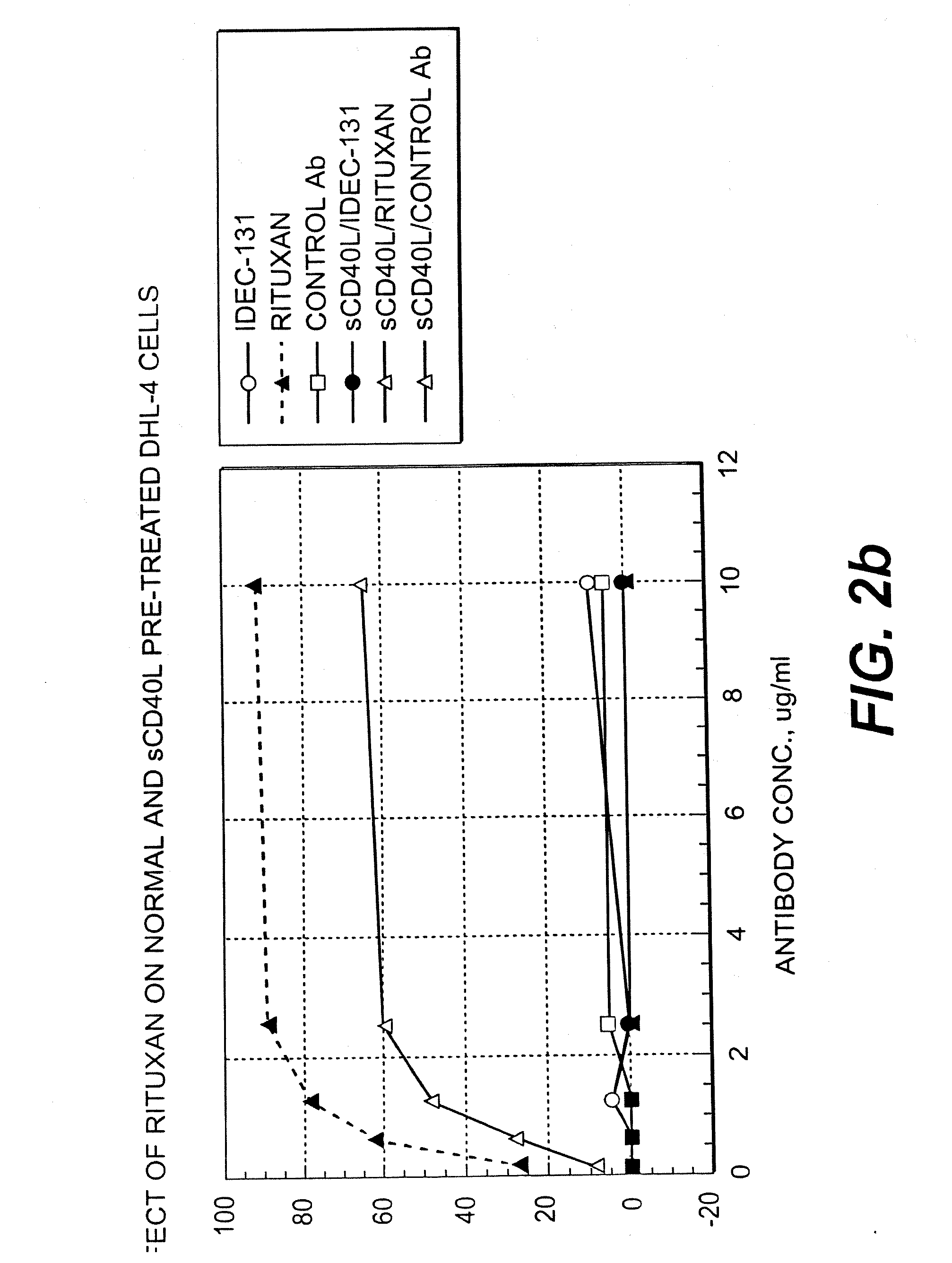
Treatment of B-cell associated diseases including autoimmune and B-cell malignancies such as leukemias, lymphomas, using the combination of an anti-CD20 antibody, preferably RITUXAN® and a radiolabeled anti-CD22 antibody, preferably an 90Y labeled humanized anti-CD22 antibody, is described. These therapeutic regimens provide for enhanced depletion of B cells, and therefore reduce the risk in B cell malignancy treatment of relapse associated with RITUXAN® and, moreover, provide for prolonged immunosuppression of B-cell immune responses, especially in the context of autoimmune diseases and transplant.
Owner:BIOGEN INC
Calicheamicin derivative-carrier conjugates
ActiveUS8153768B2Strong specificityReduce aggregationOrganic active ingredientsPowder deliveryAntibody conjugateCytotoxic drug
Methods for preparing monomeric cytotoxic drug / carrier conjugates with a drug loading significantly higher than in previously reported procedures and with decreased aggregation and low conjugate fraction (LCF) are described. Cytotoxic drug derivative / antibody conjugates, compositions comprising the conjugates and uses of the conjugates are also described. Monomeric calicheamicin derivative / anti-CD22 antibody conjugates, compositions comprising the conjugates and uses of the conjugates are also described.
Owner:WYETH HOLDINGS LLC
Camptothecin Conjugates of Anti-CD22 Antibodies for Treatment of B Cell Diseases
ActiveUS20110305631A1Increase the number ofNervous disorderPeptide/protein ingredientsCD20Autoimmune condition
Disclosed herein are compositions and methods of use comprising combinations of anti-CD22 antibodies with a therapeutic agent. The therapeutic agent may be attached to the anti-CD22 antibody or may be separately administered, either before, simultaneously with or after the anti-CD22 antibody. In preferred embodiments, the therapeutic agent is an antibody or fragment thereof that binds to an antigen different from CD22, such as CD19, CD20, CD21, CD22, CD23, CD37, CD40, CD40L, CD52, CD80 and HLA-DR. However, the therapeutic agent may an immunomodulator, a cytokine, a toxin or other therapeutic agent known in the art. More preferably, the anti-CD22 antibody is part of a DNL complex, such as a hexavalent DNL complex. Most preferably, combination therapy with the anti-CD22 antibody or fragment and the therapeutic agent is more effective than the antibody alone, the therapeutic agent alone, or the combination of anti-CD22 antibody and therapeutic agent that are not conjugated to each other. Administration of the anti-CD22 antibody and therapeutic agent induces apoptosis and cell death of target cells in diseases such as B-cell lymphomas or leukemias, autoimmune disease or immune dysfunction disease.
Owner:IMMUNOMEDICS INC
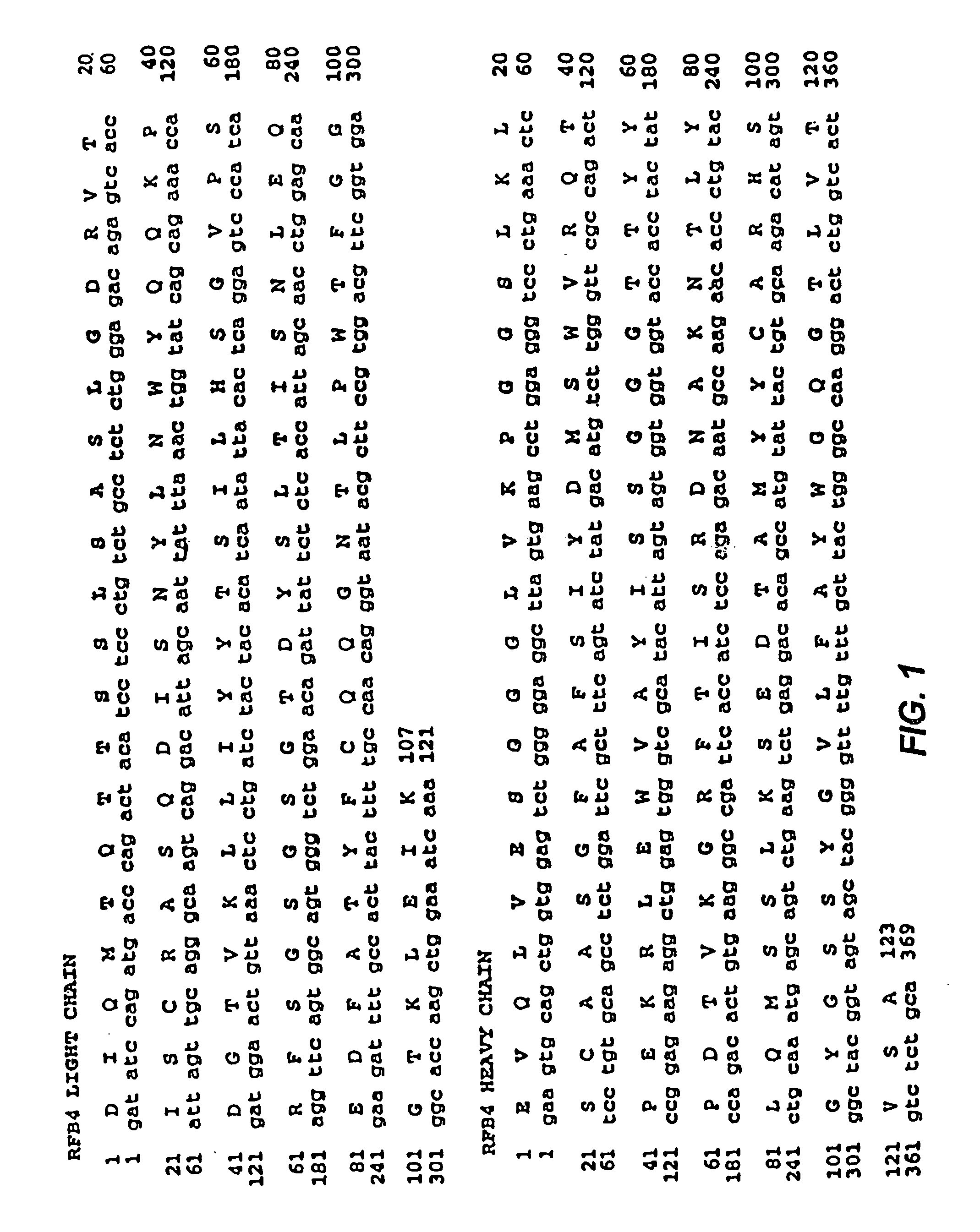
Mutated anti-CD22 antibodies with increased affinity to CD22-expressing leukemia cells
ActiveUS7355012B2Snake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenBacteroides
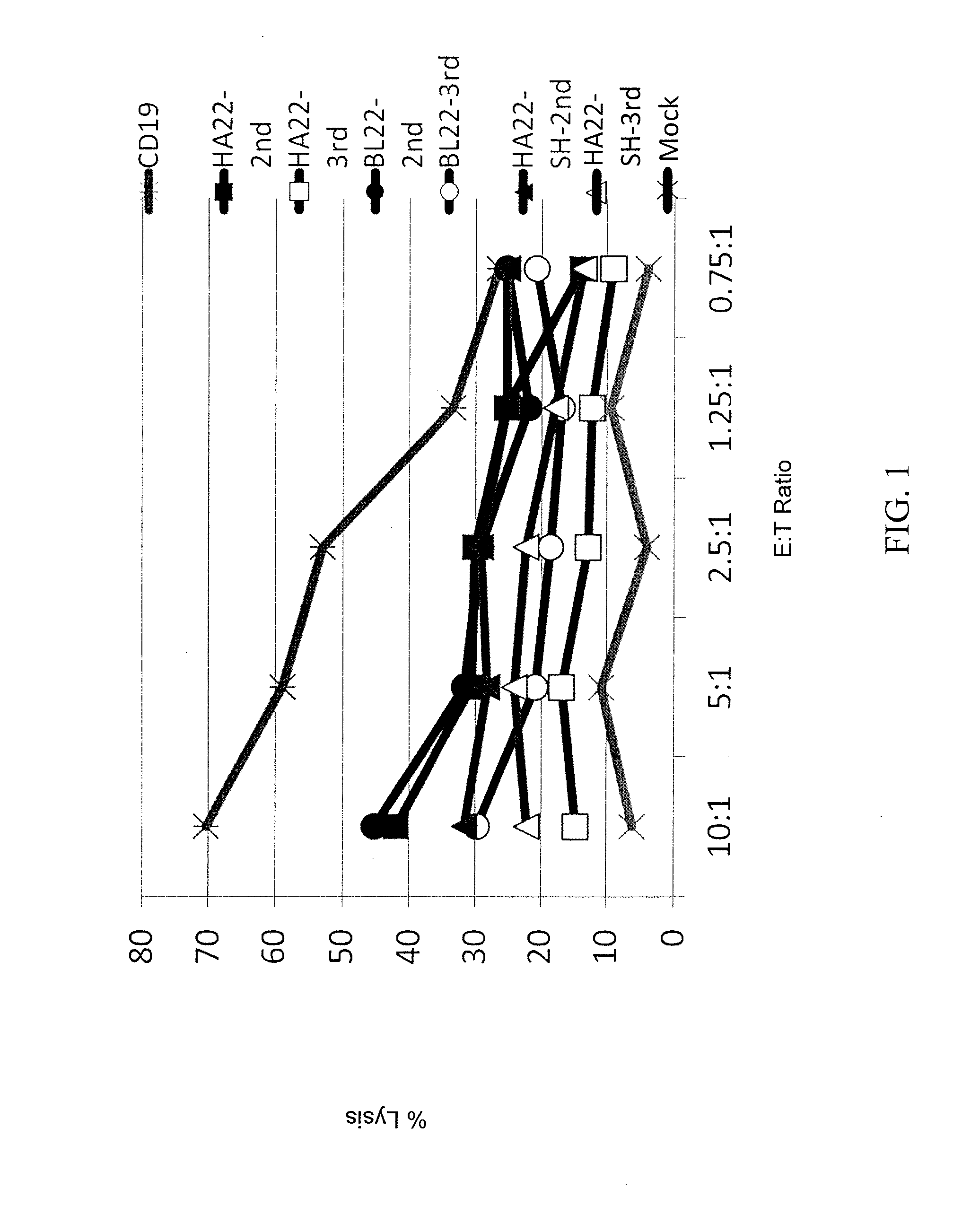
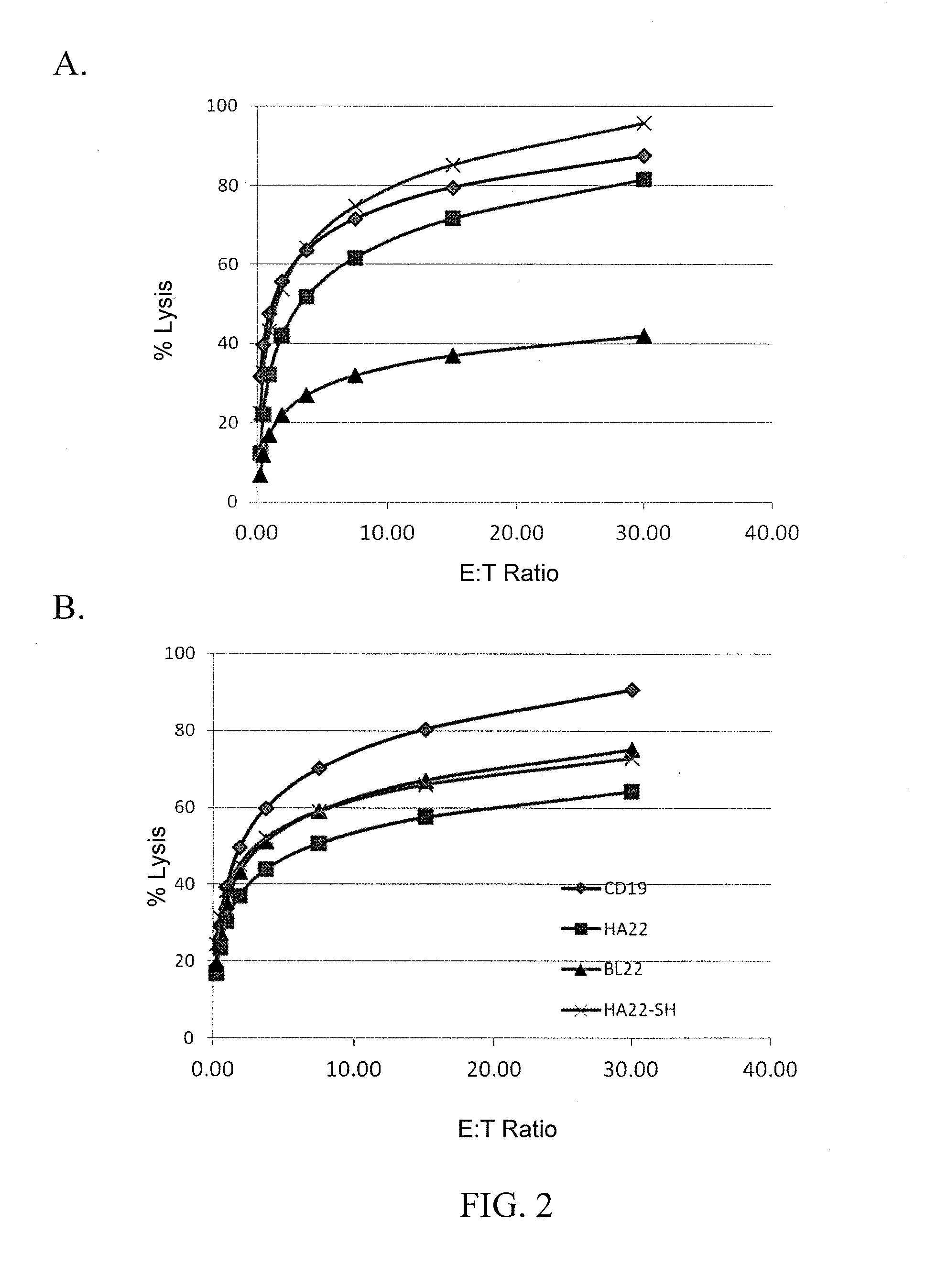
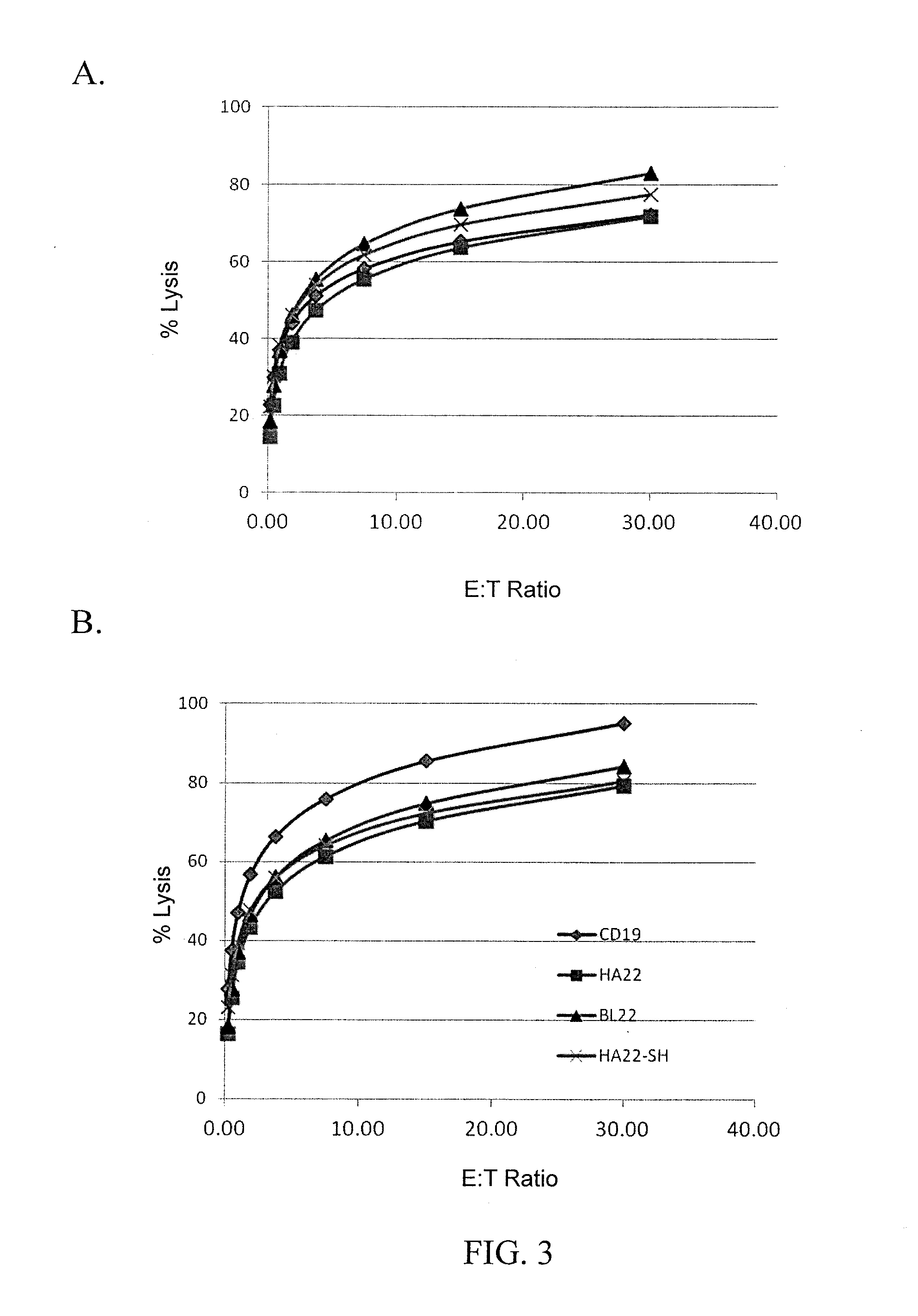
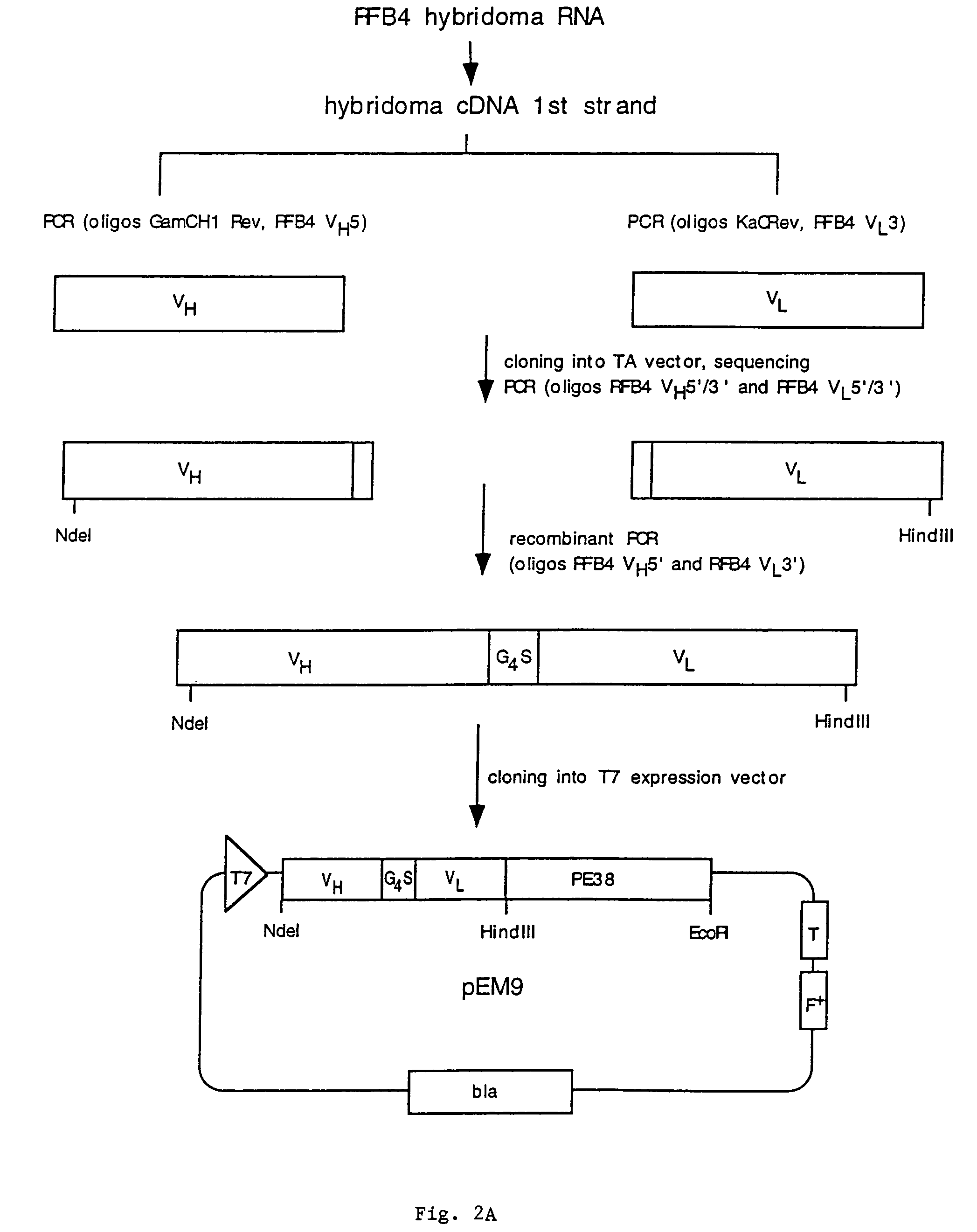
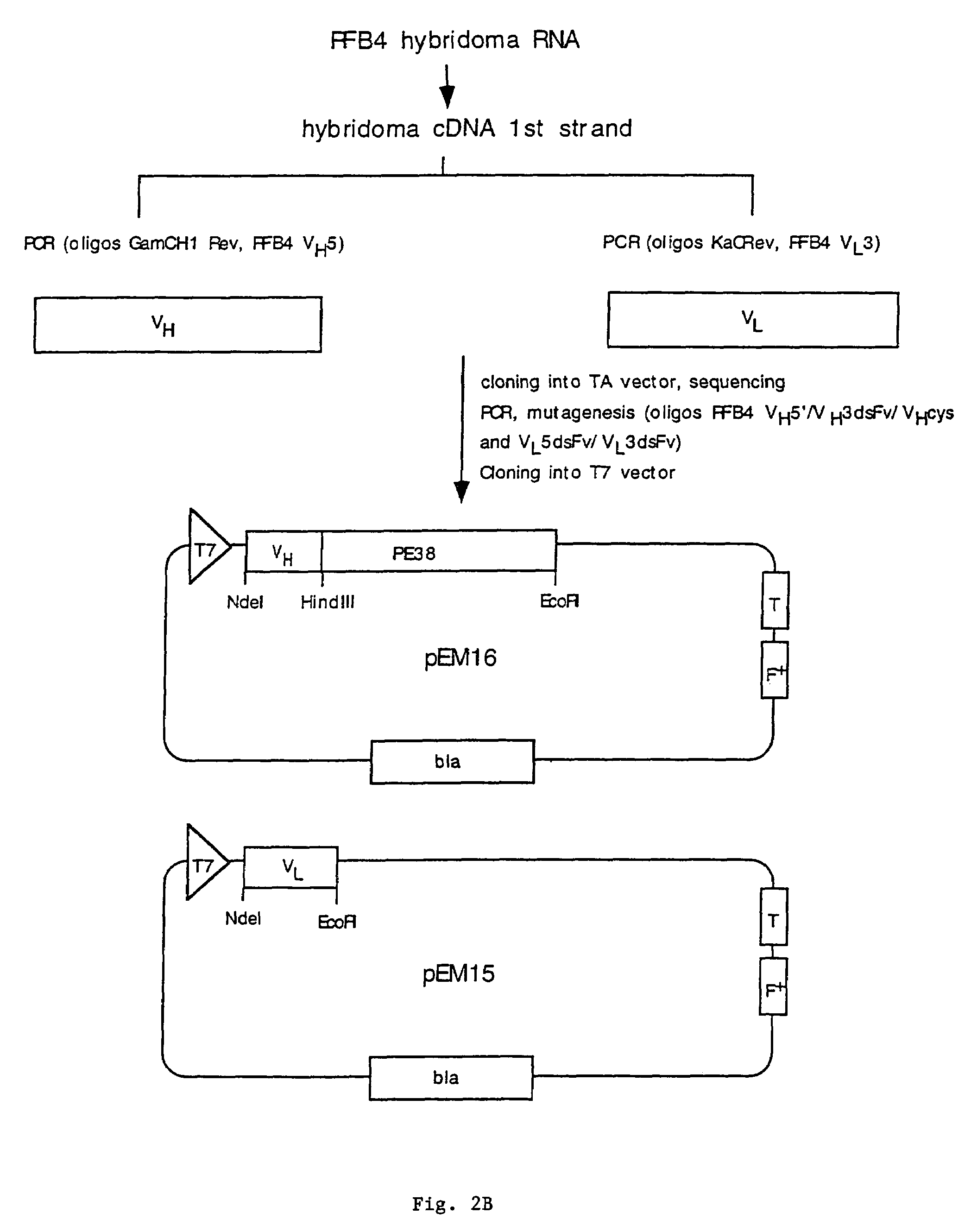
Recombinant immunotoxins are fusion proteins composed of the Fv domains of antibodies fused to bacterial or plant toxins. RFB4 (Fv)-PE38 is an immunotoxin that targets CD22 expressed on B cells and B cell malignancies. The present invention provides antibodies and antibody fragments that have improved ability to bind the CD22 antigen of B cells and B cell malignancies compared to RFB4. Immunotoxins made with the antibodies and antibody fragments of the invention have improved cytotoxicity to CD22-expressing cancer cells. Compositions that incorporate these antibodies into chimeric immunotoxin molecules that can be used in medicaments and methods for inhibiting the growth and proliferation of leukemia and lymphoma cells.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES
Anti-cd22 chimeric antigen receptors
The disclosure provides a chimeric antigen receptor (CAR) comprising a) an antigen binding domain of HA22, a transmembrane domain, and an intracellular T cell signaling domain; or b) an antigen binding domain of BL22, a transmembrane domain, and an intracellular T cell signaling domain comprising CD28 and / or CD137. Nucleic acids, recombinant expression vectors, host cells, populations of cells, antibodies, or antigen binding portions thereof, and pharmaceutical compositions relating to the CARs are disclosed. Methods of detecting the presence of cancer in a mammal and methods of treating or preventing cancer in a mammal are also disclosed.
Owner:UNITED STATES OF AMERICA
Recombinant antibodies and immunoconjugates targeted to CD-22 bearing cells and tumors
InactiveUS7541034B1Growth inhibitionBacteriaPeptide/protein ingredientsPseudomonas aeruginosa exotoxin APseudomonas
Methods and compositions relating to recombinant anti-CD22 antibodies with high binding affinity, and immunoconjugates comprising the anti-CD22 antibody linked to a therapeutic agent such as a Pseudomonas exotoxin or a detectable label. The invention provides diagnostic methods, and means to inhibit the growth of malignant B cells.
Owner:UNITED STATES OF AMERICAN AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES
Treatment of B-cell associated diseases such as malignancies and autoimmune diseases using a cold anti-CD20 antibody/radiolabeled anti-CD22 antibody combination
InactiveUS20050112060A1Enhanced killing and depletionPrevent and inhibit relapseRadioactive preparation carriersAntibody ingredientsAutoimmune conditionRegimen
Owner:BIOGEN INC
Treatment of B cell malignancies using combination of B cell depleting antibody and immune modulating antibody related applications
InactiveUS20050123540A1Radioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:IDEC PHARM CORP +1
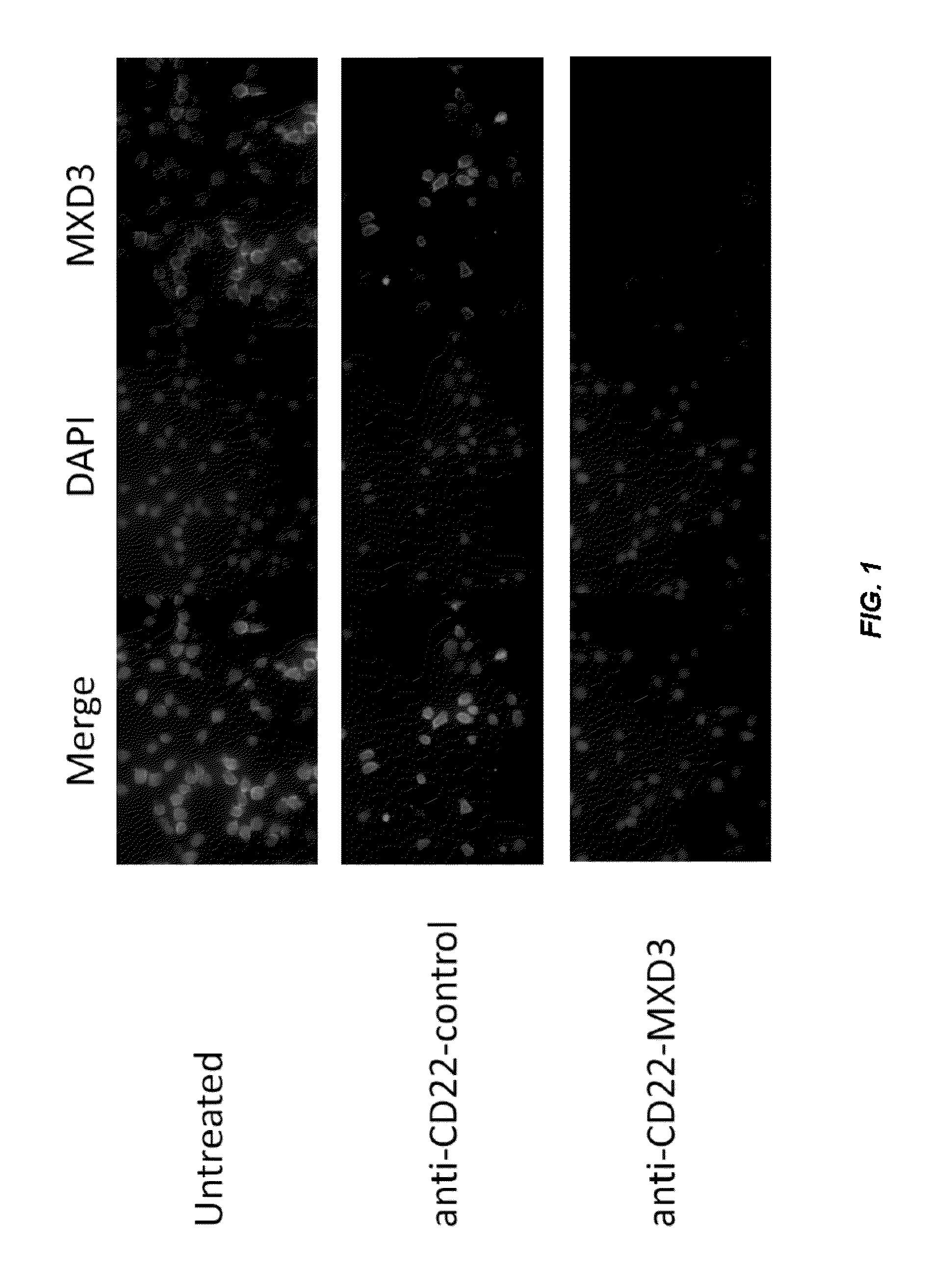
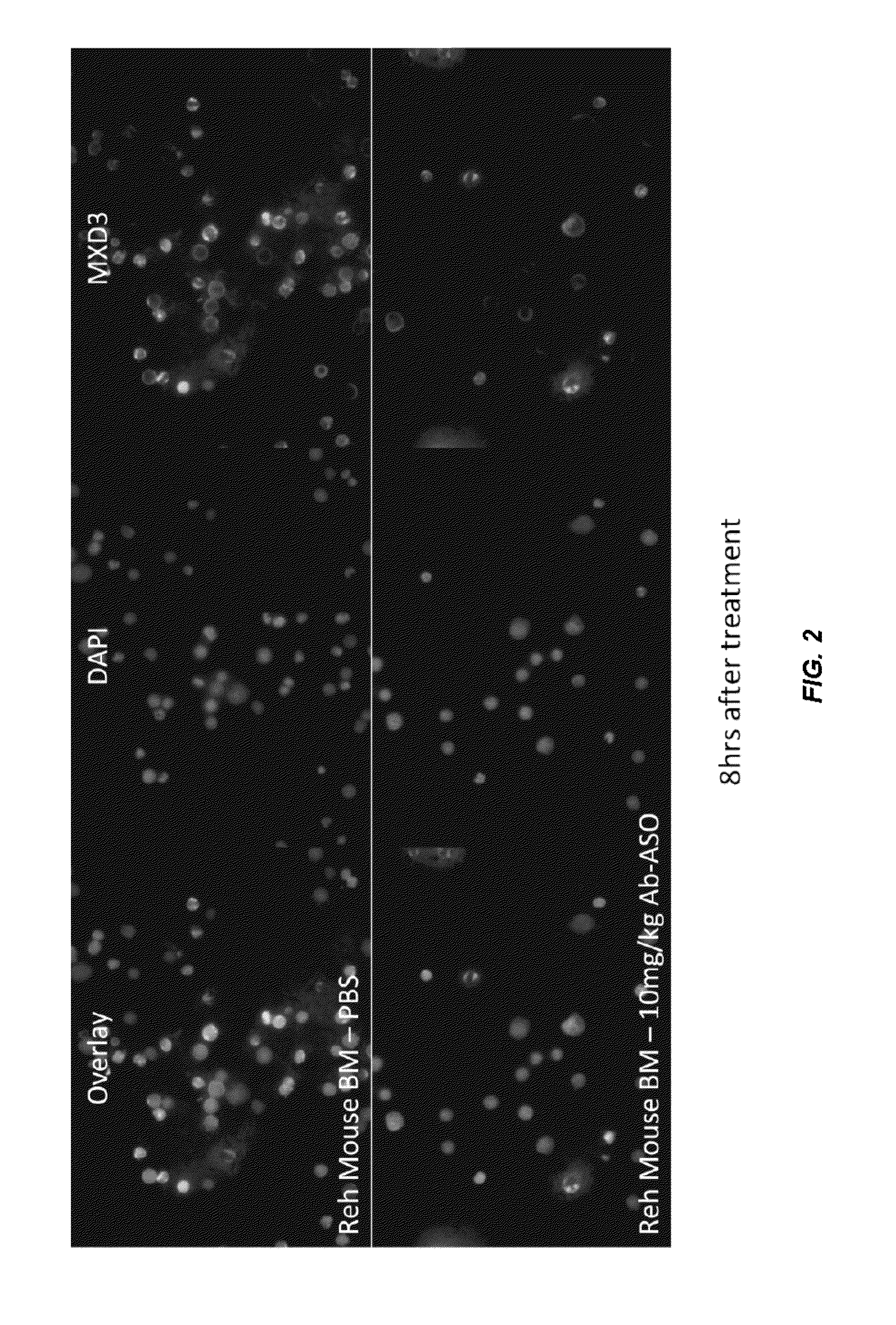
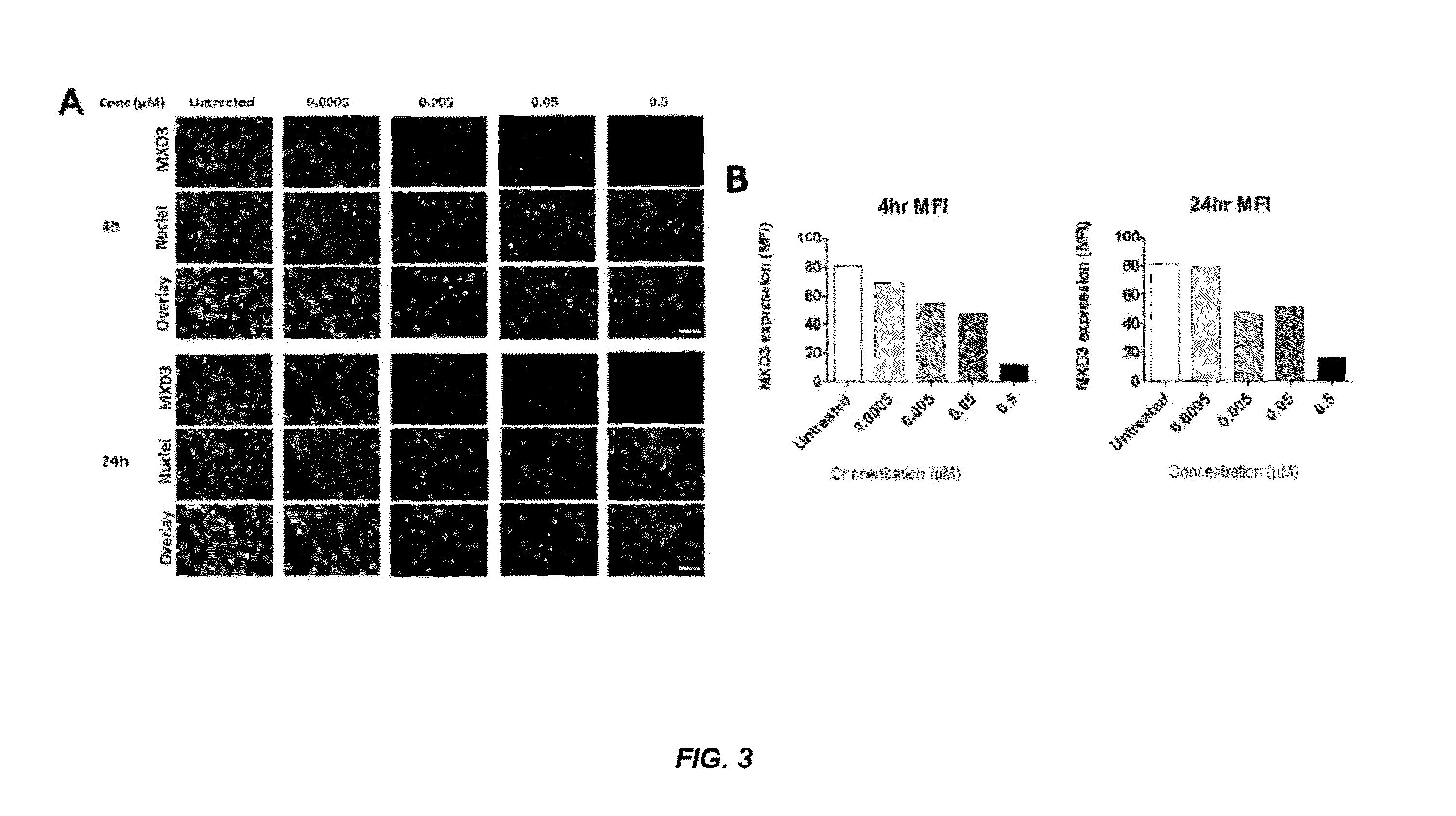
Antisense compounds and uses thereof
ActiveUS20160090598A1Reduce expressionOrganic active ingredientsSpecial deliveryNucleic acid hybridisationOligonucleotide
The present disclosure provides compounds comprising modified oligonucleotides and anti-CD22 antibodies. Certain such modified oligonucleotides conjugated to anti-CD22 antibodies are useful for hybridizing to a complementary nucleic acid, including but not limited, to nucleic acids in a cell. In certain embodiments, hybridization results in modulation of the amount activity or expression of the target nucleic acid in a cell.
Owner:IONIS PHARMA INC +1
Immunoregulatory Antibodies and Uses Thereof
InactiveUS20070009519A1Convenient treatmentIn-vivo radioactive preparationsPharmaceutical containersCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
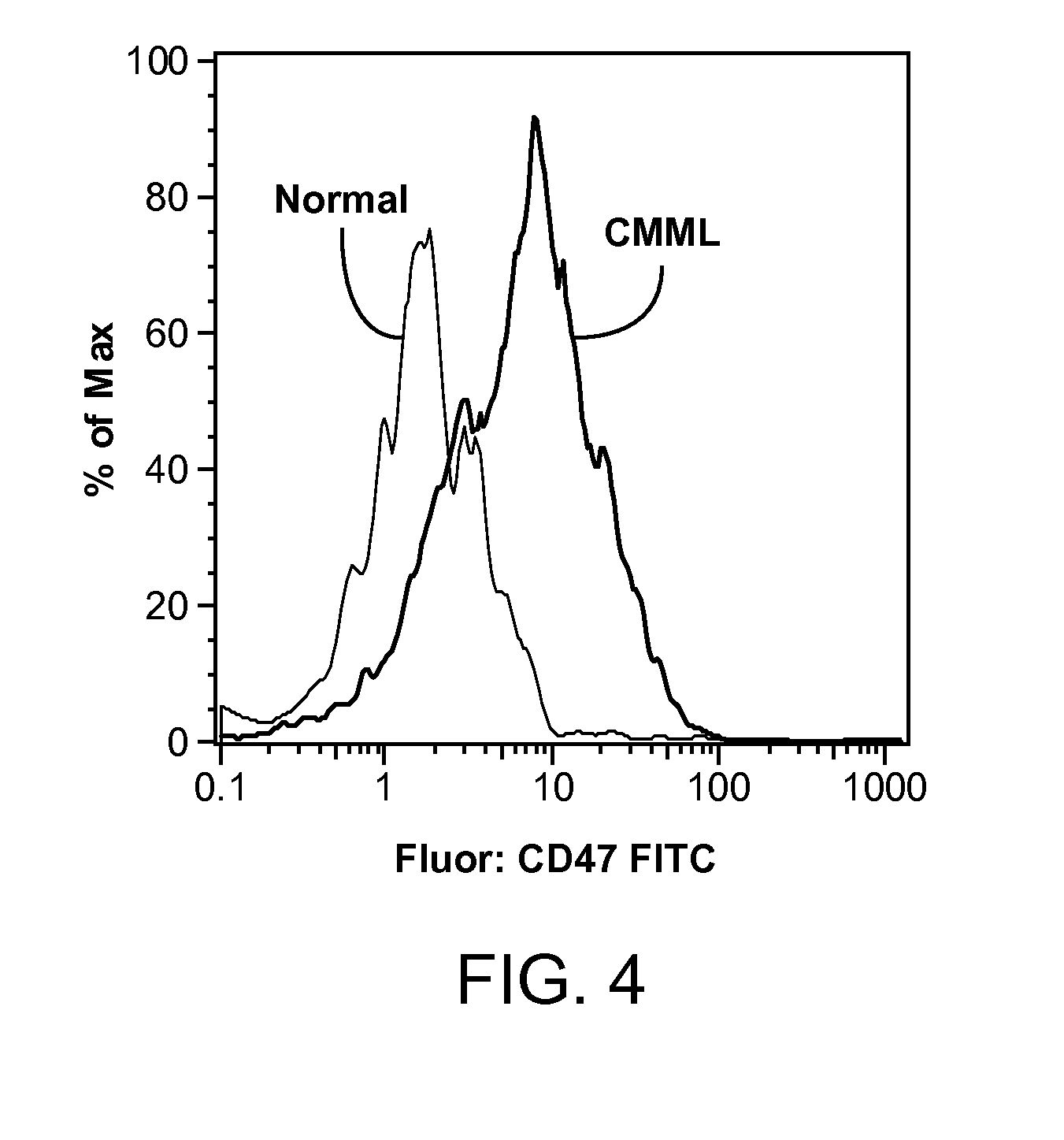
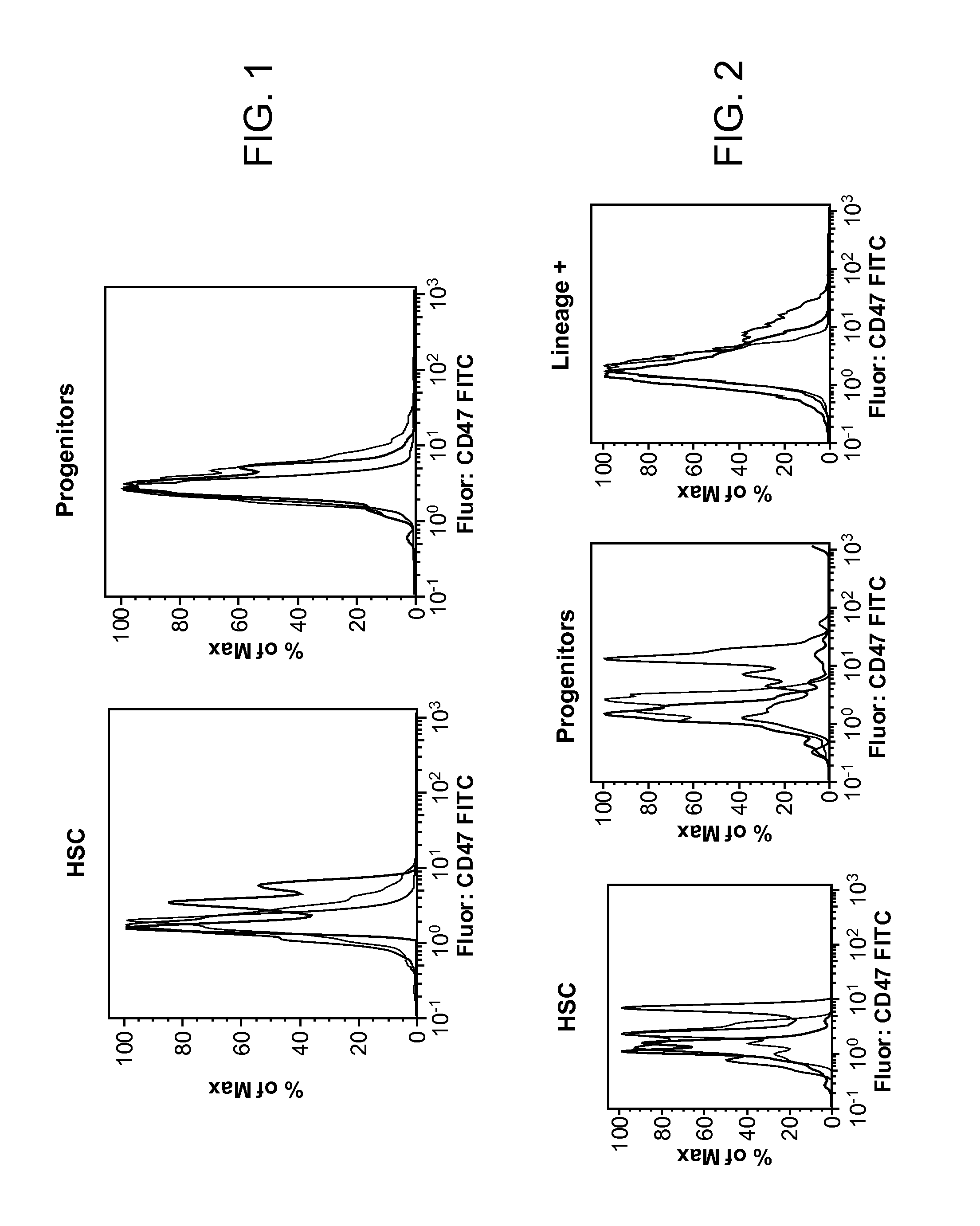
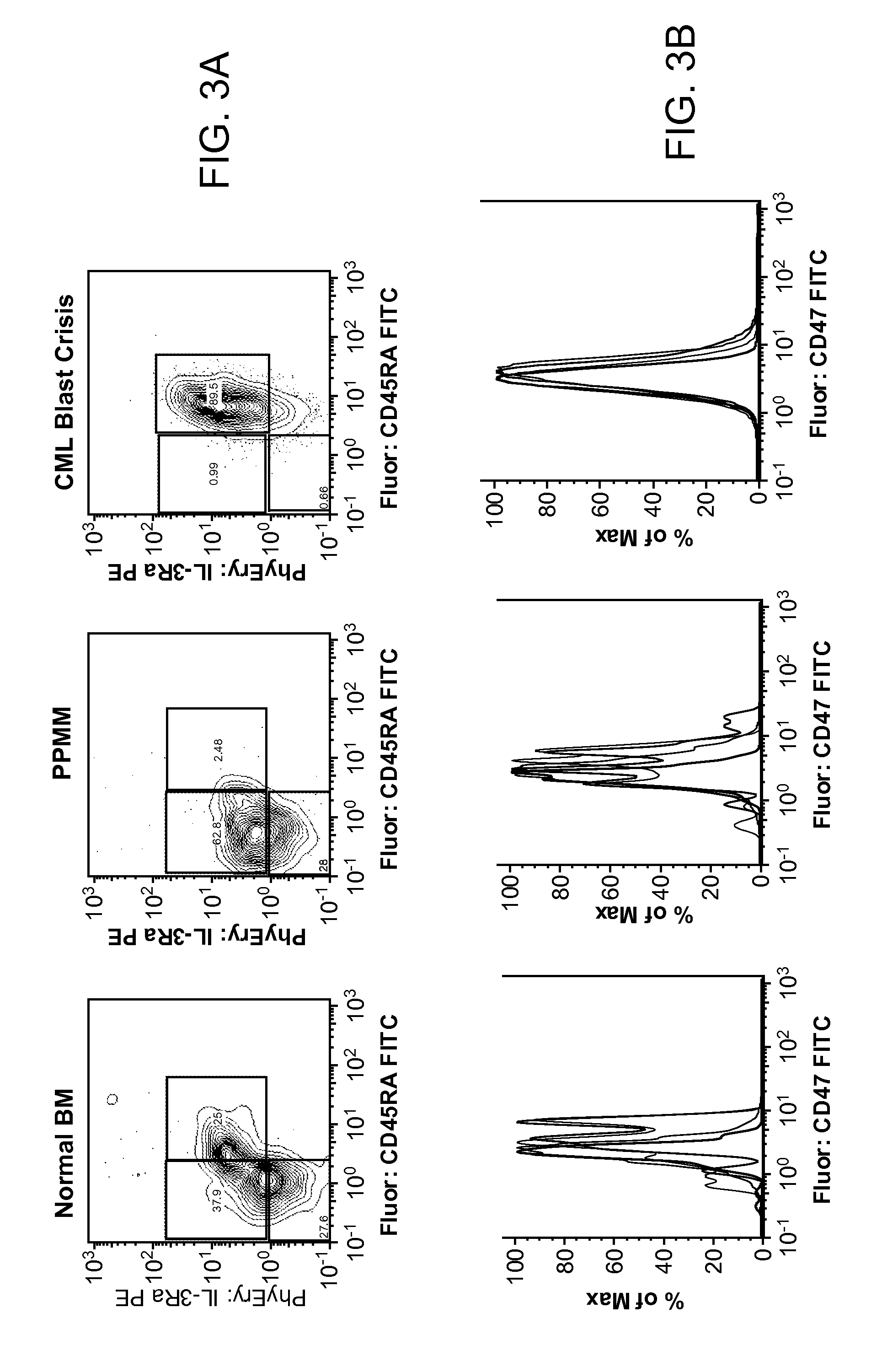
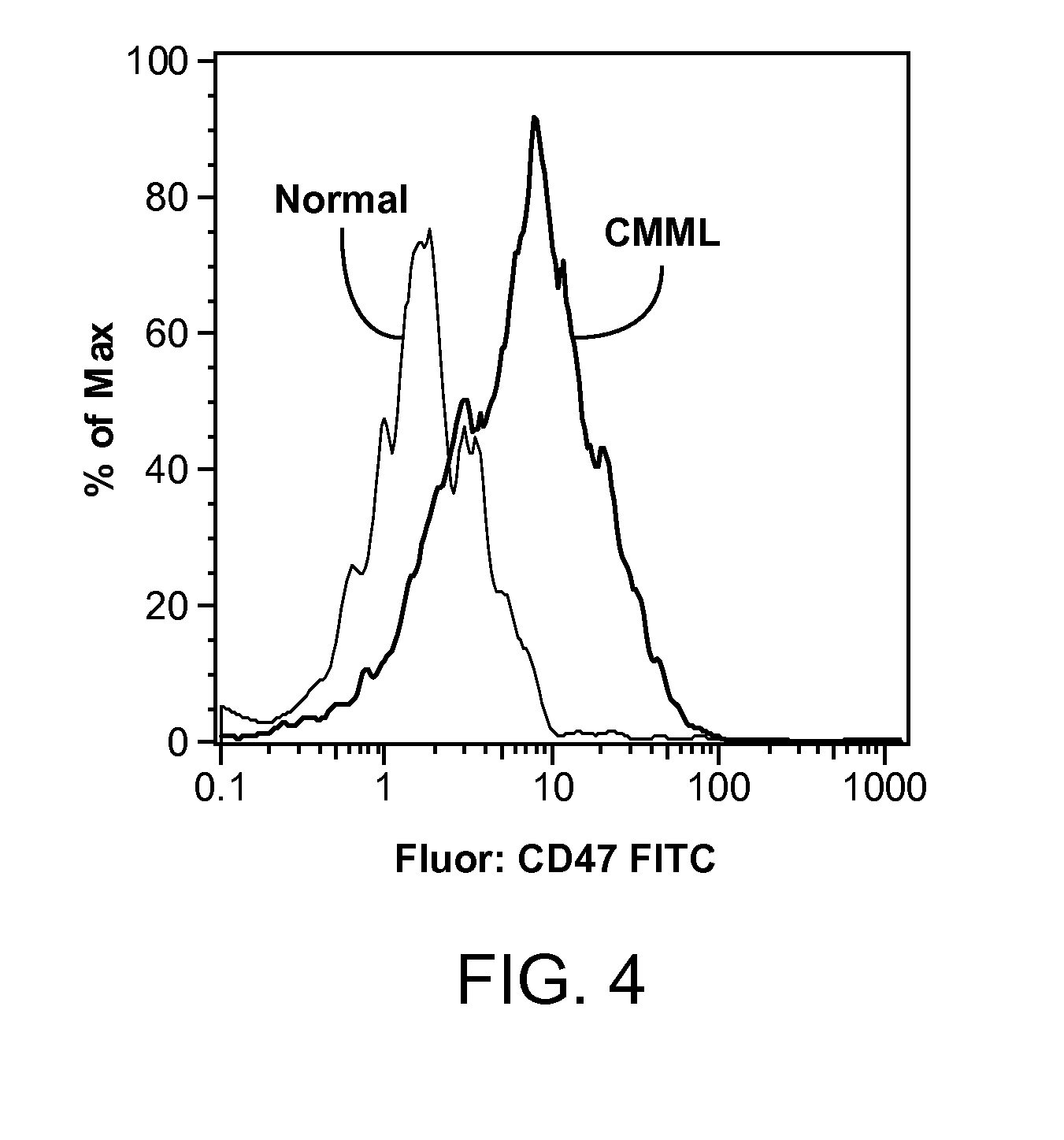
Synergistic Anti-CD47 Therapy for Hematologic Cancers
ActiveUS20120282174A1Immunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersSurface markerCD20
Methods are provided for treatment of hematologic cancers, particularly lymphomas and leukemias, including without limitation myelogenous and lymphocytic leukemias. A combination of antibodies specific for CD47; and specific for a cancer associated cell surface marker are administered to the patient, and provide for a synergistic decrease in cancer cell burden. The combination of antibodies may comprise a plurality of monospecific antibodies, or a bispecific or multispecific antibody. Markers of interest include without limitation, CD20, CD22, CD52, CD33; CD96; CD44; CD123; CD97; CD99; PTHR2; and HAVCR2.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
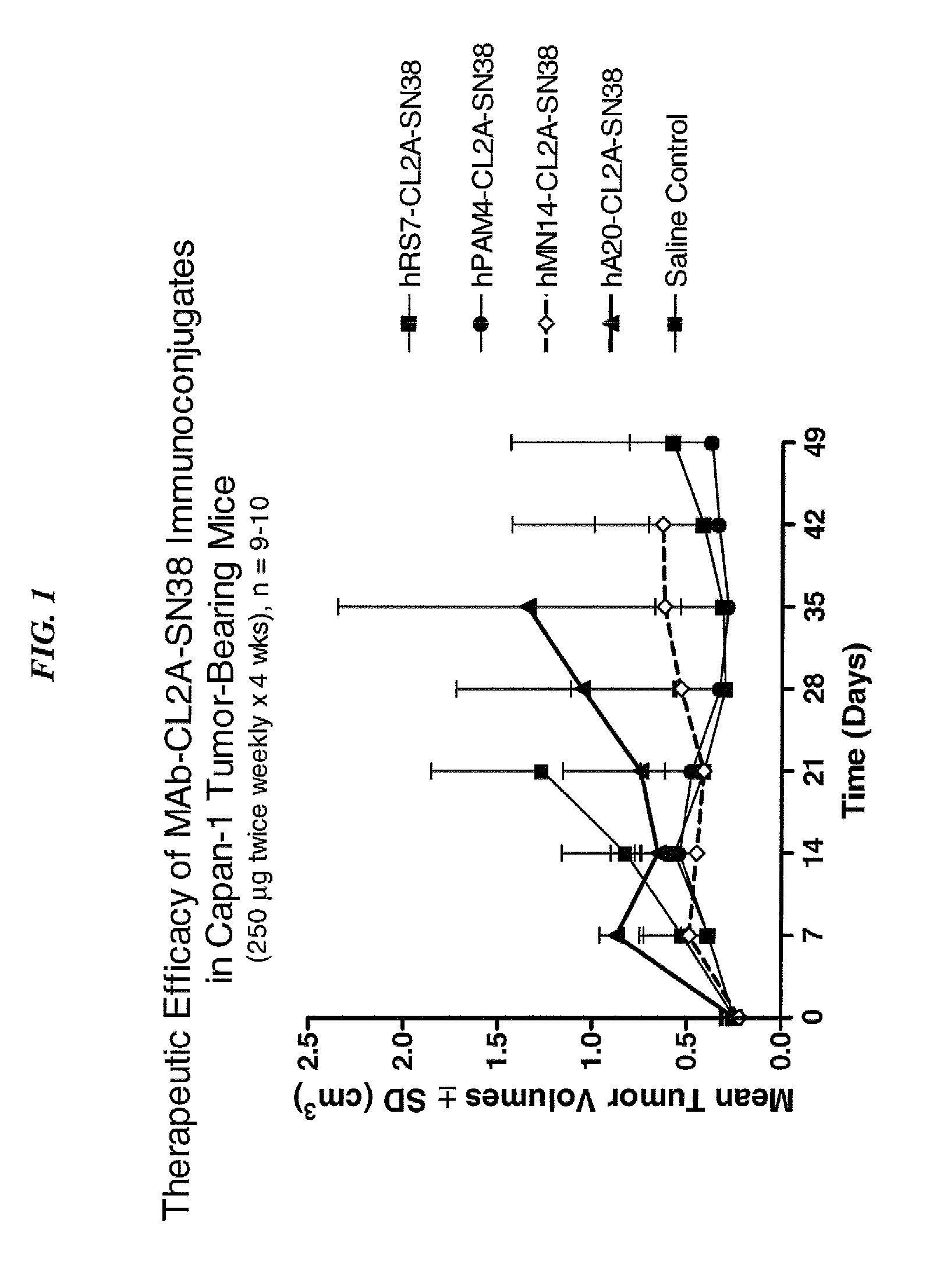
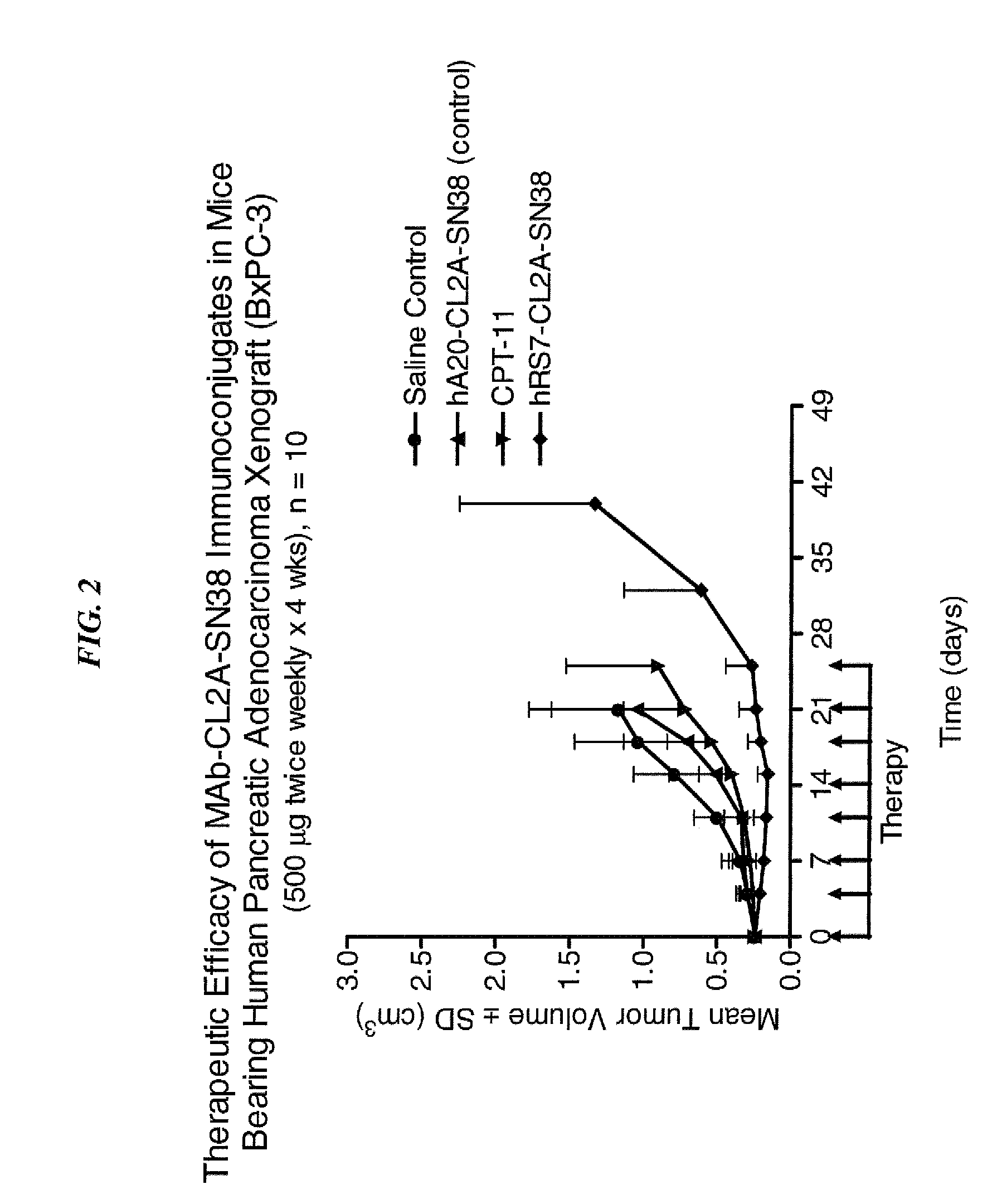
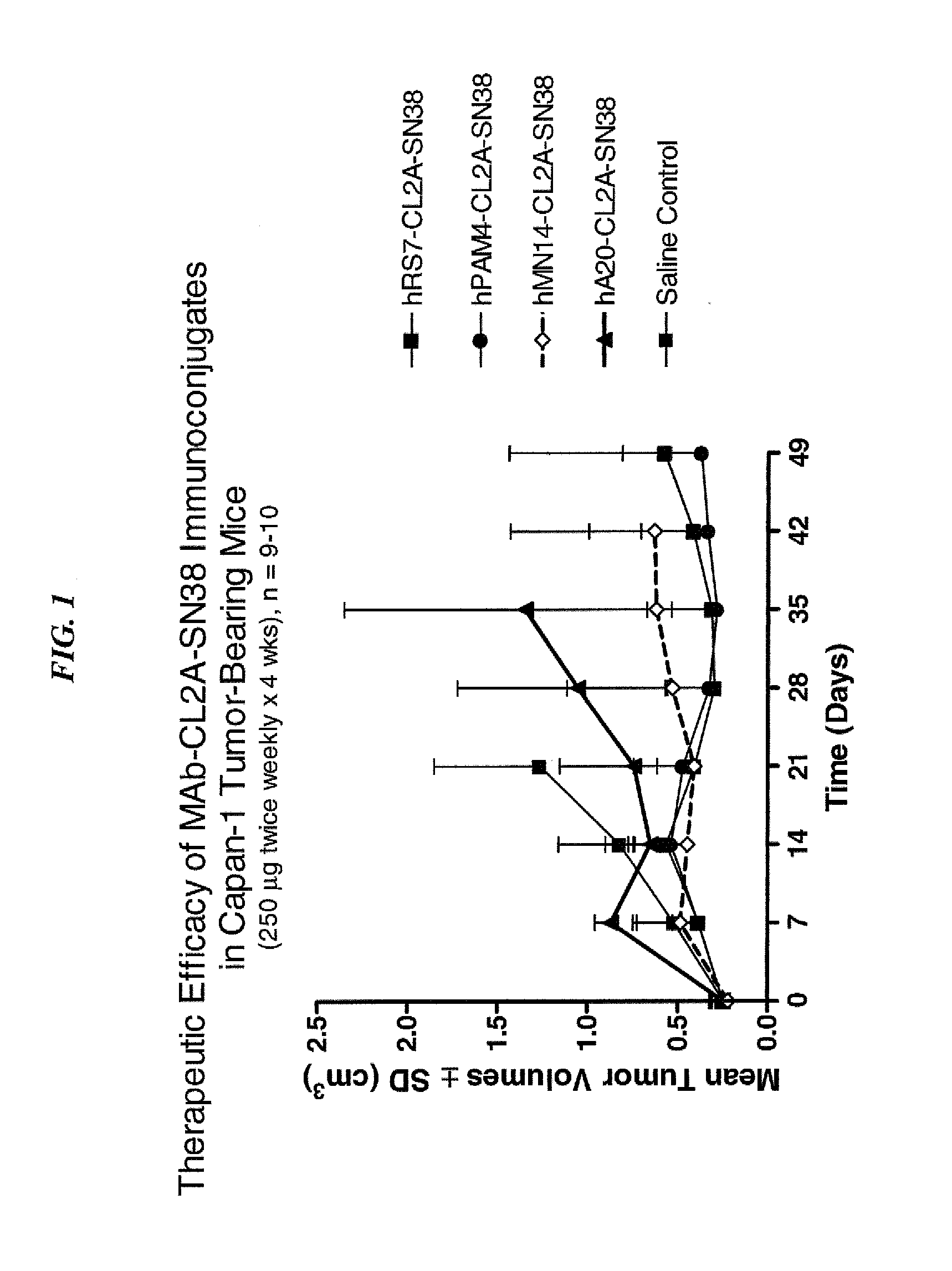
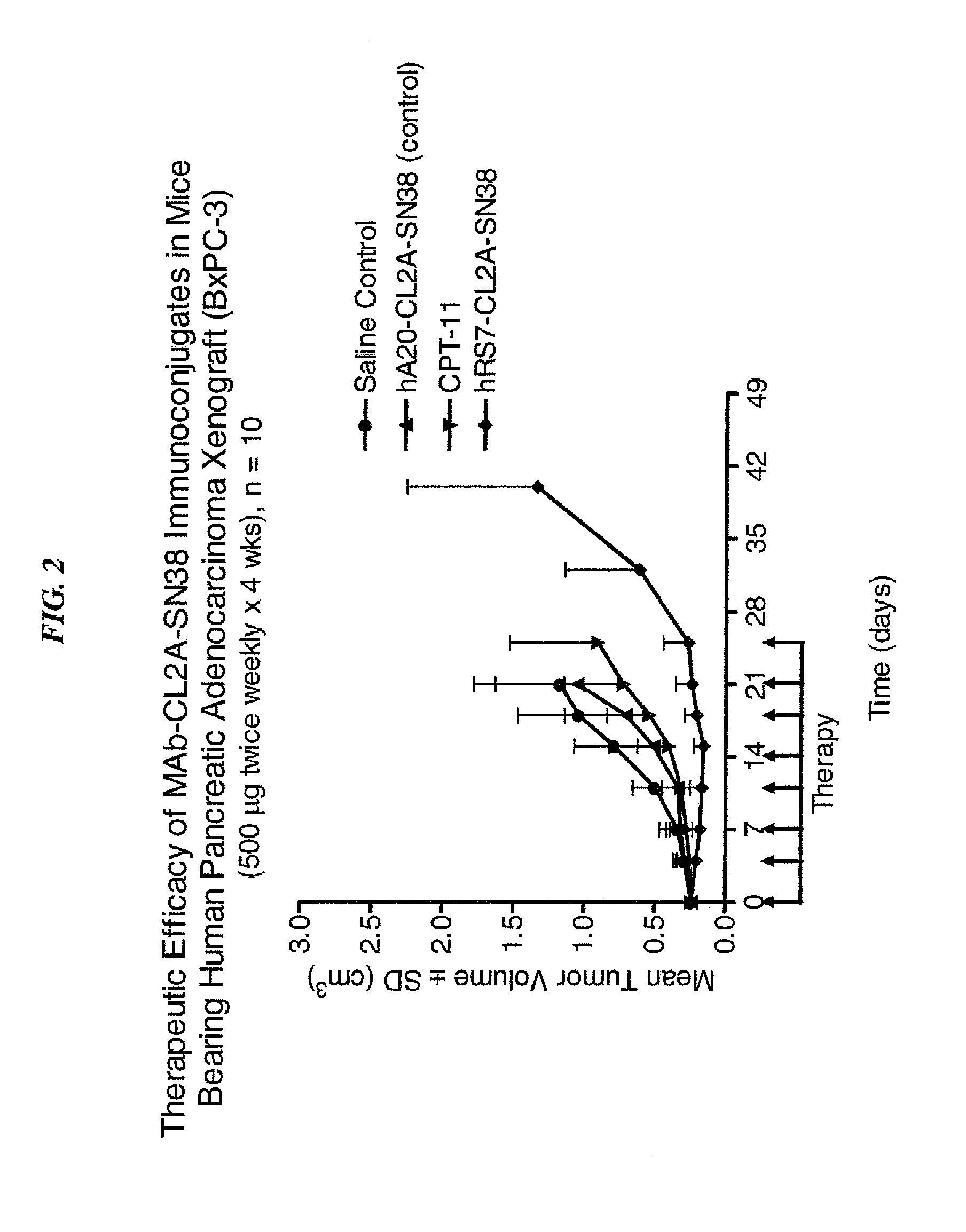
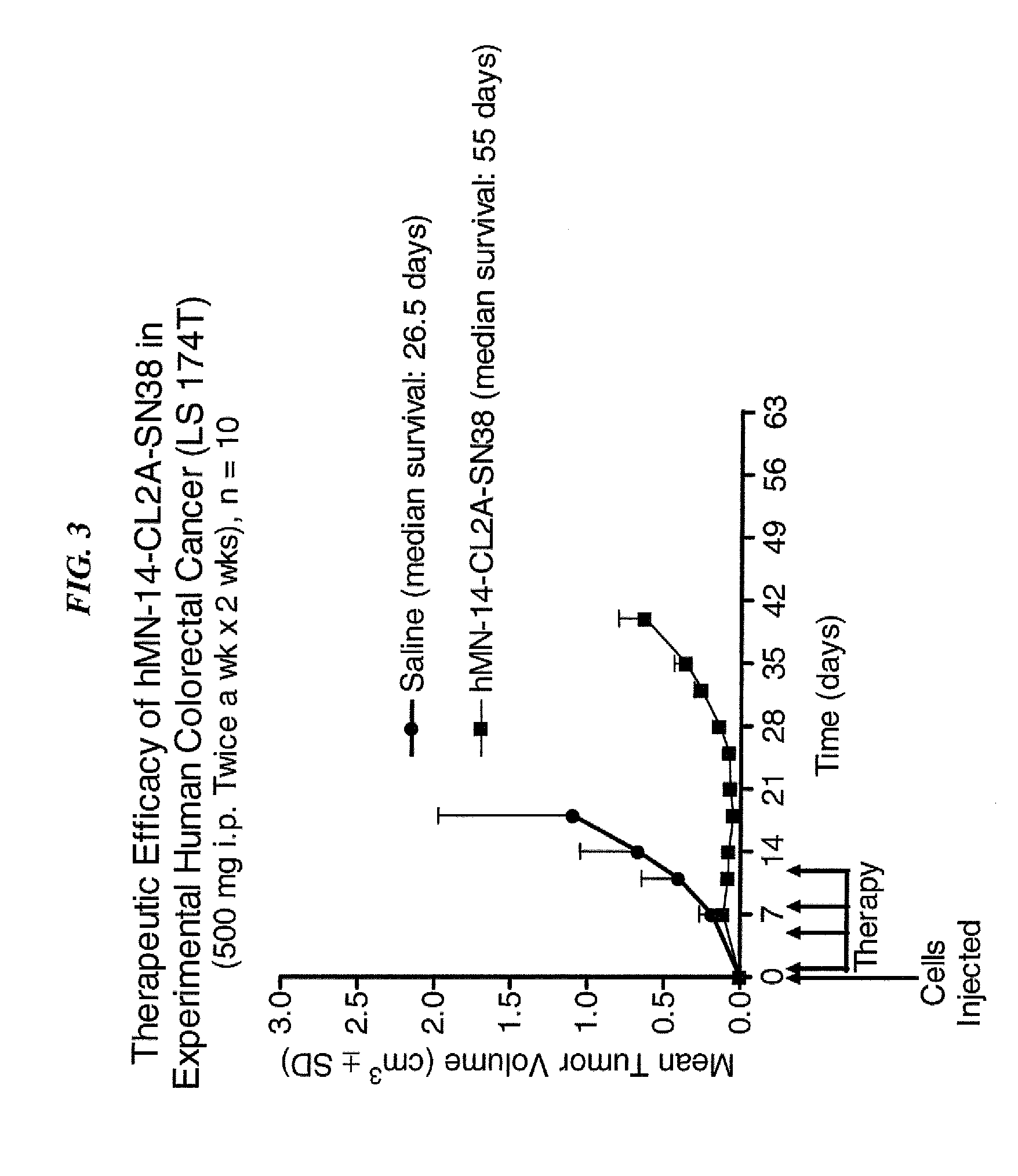
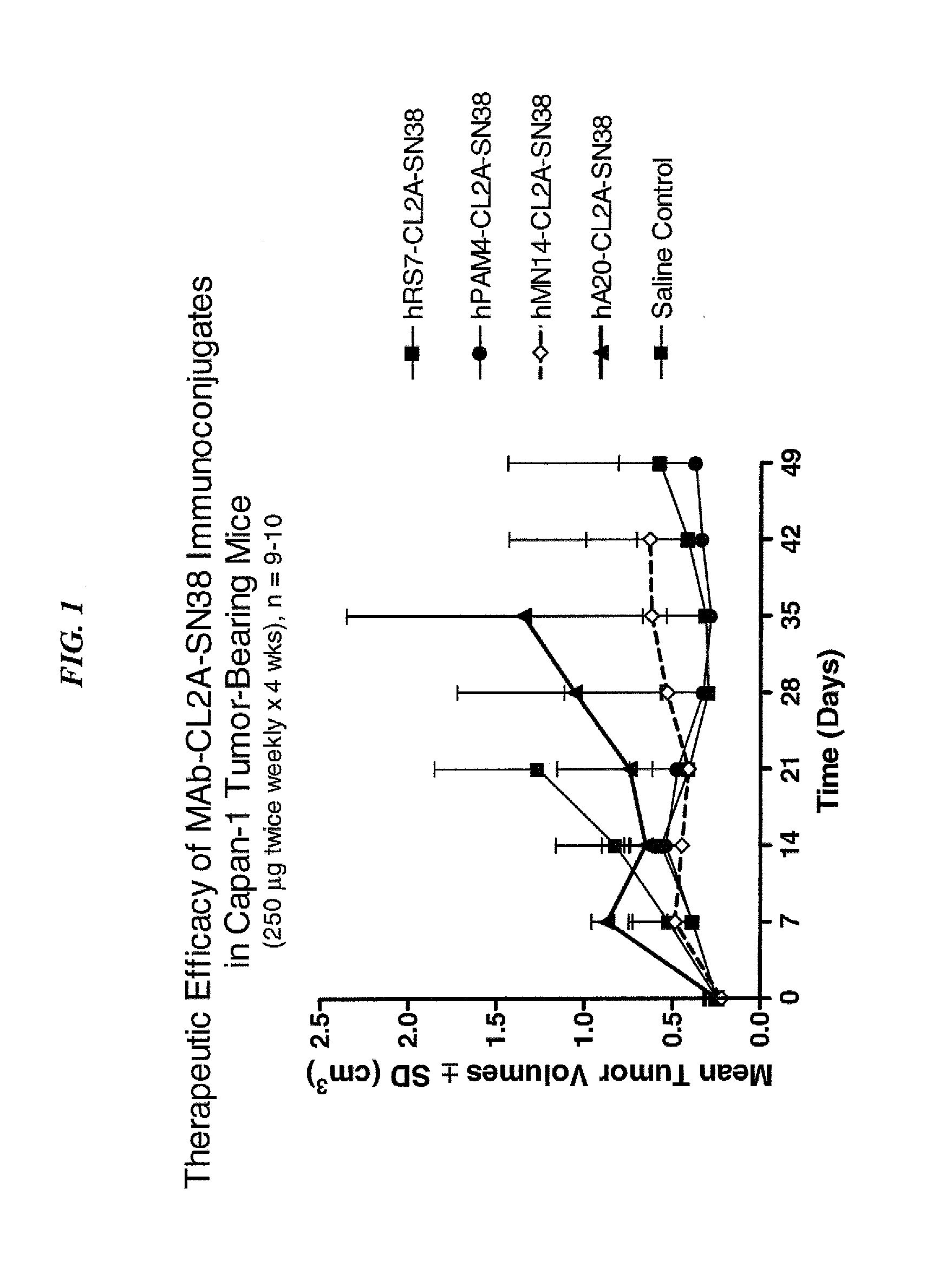
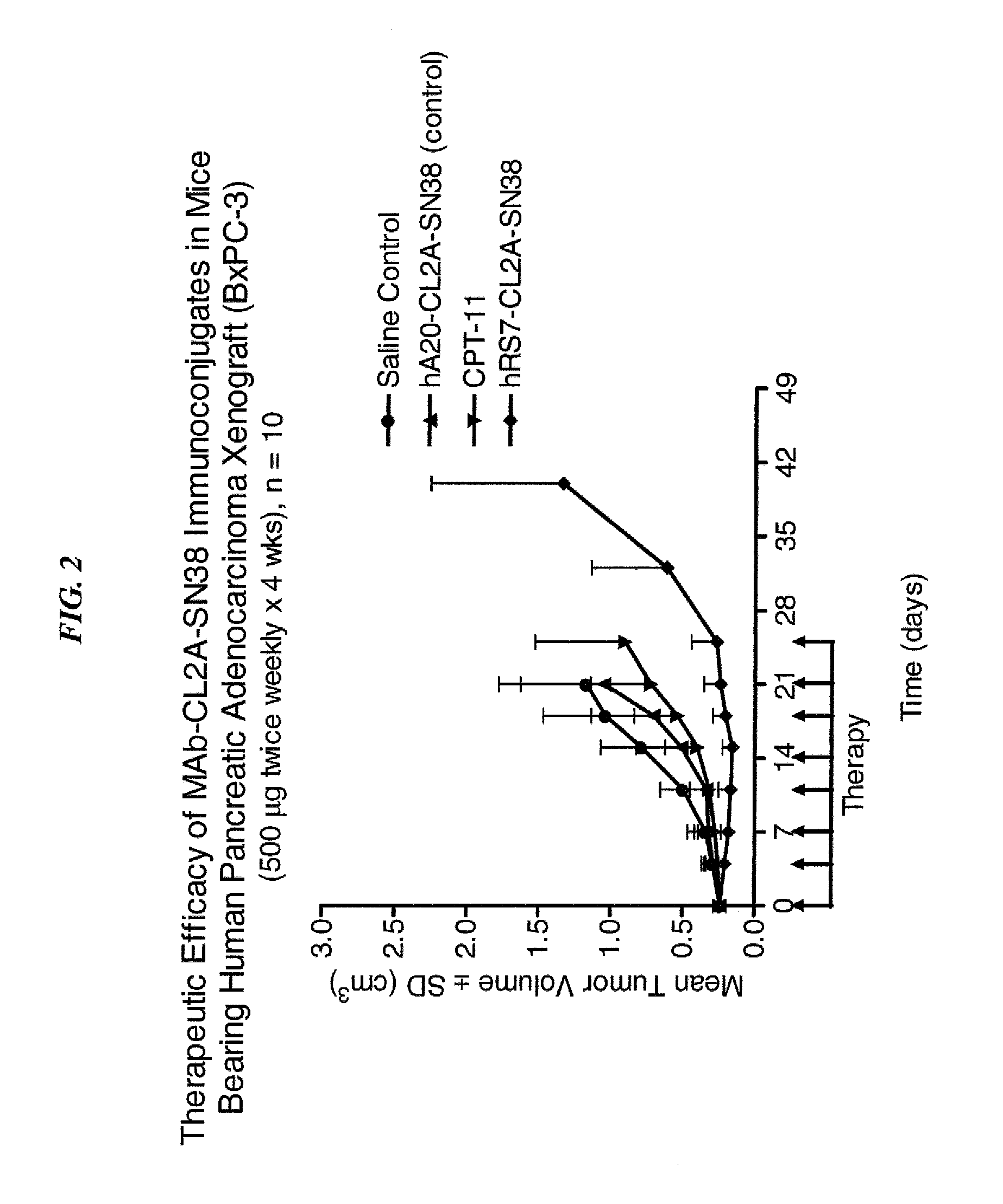
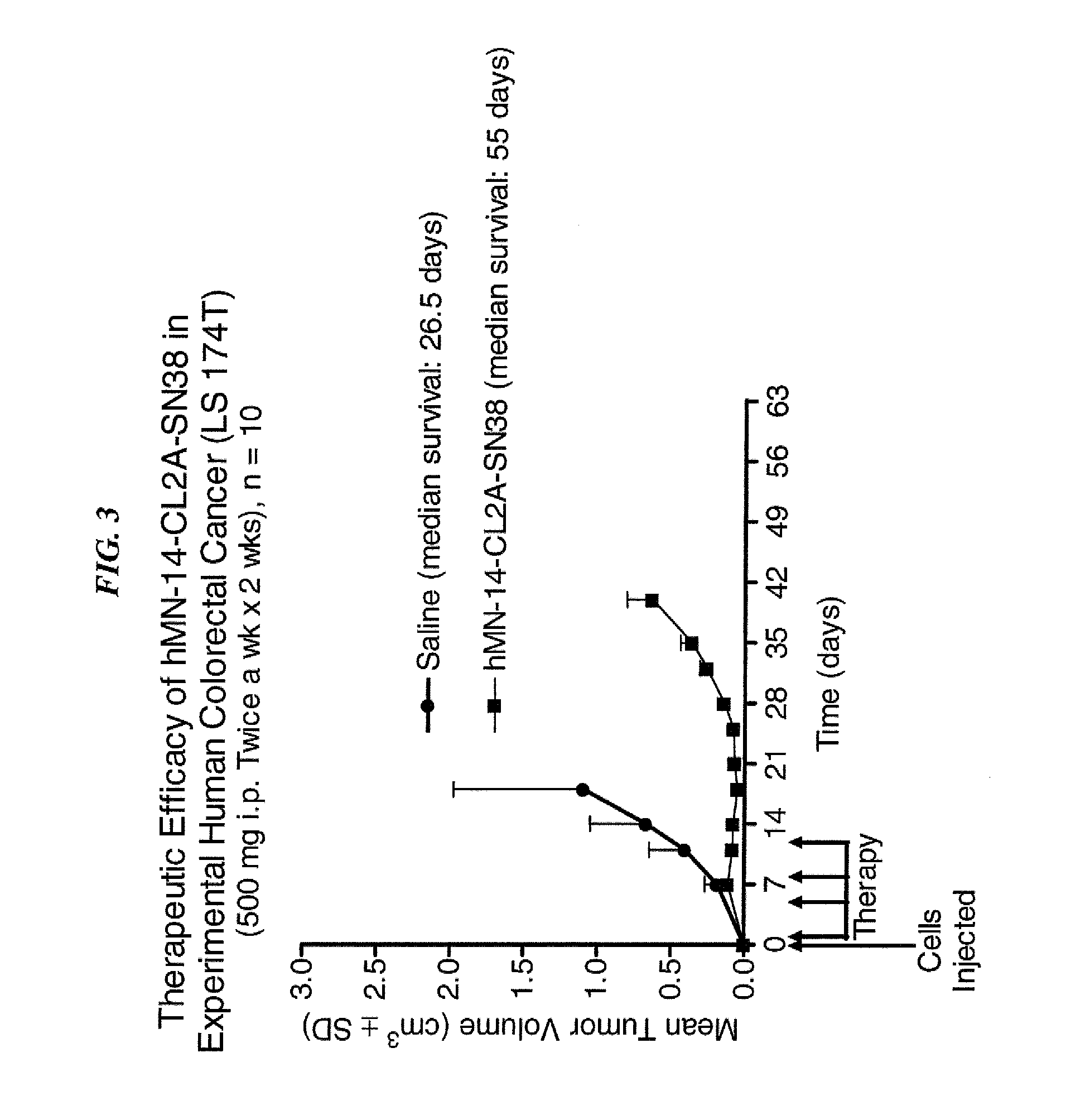
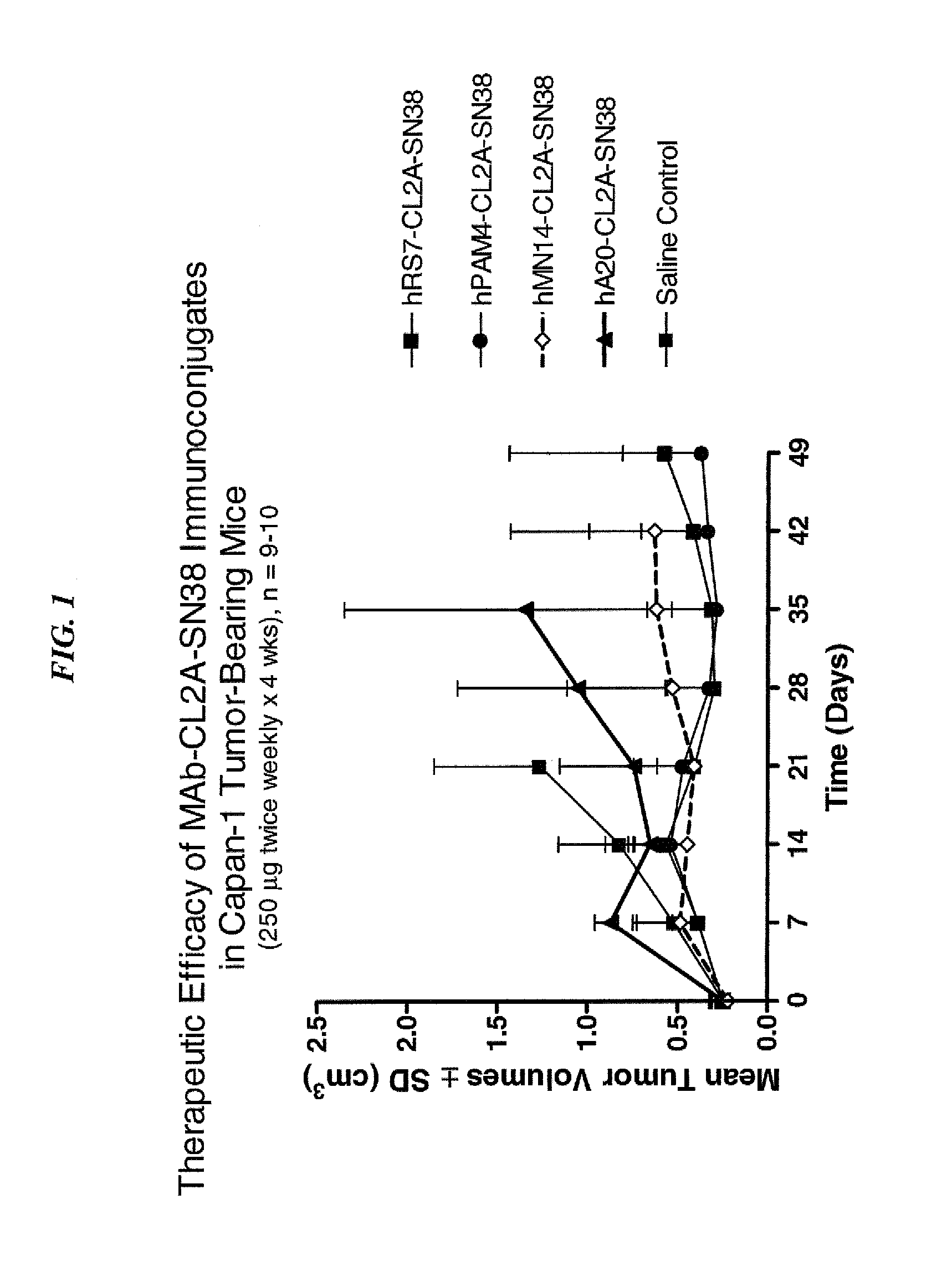
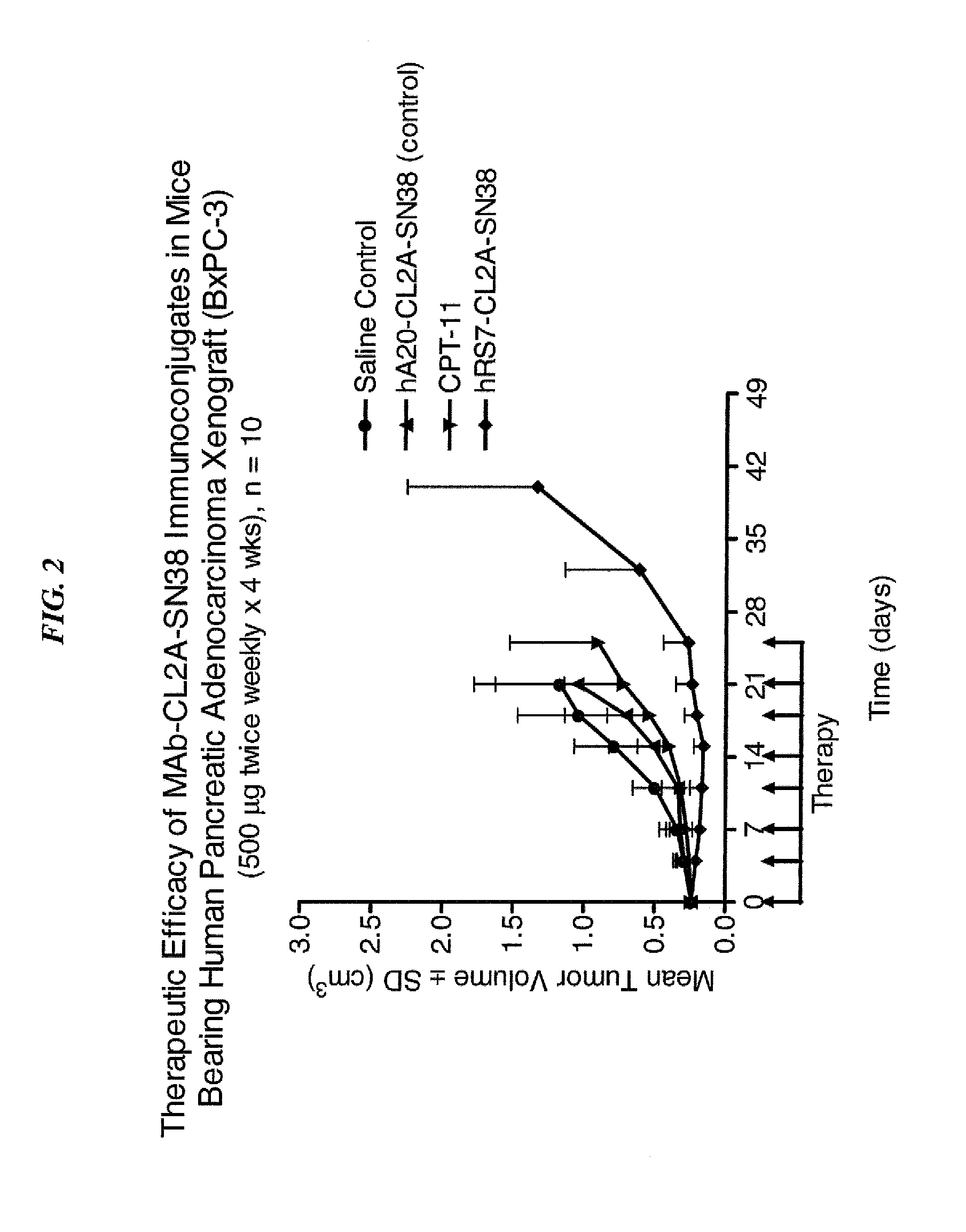
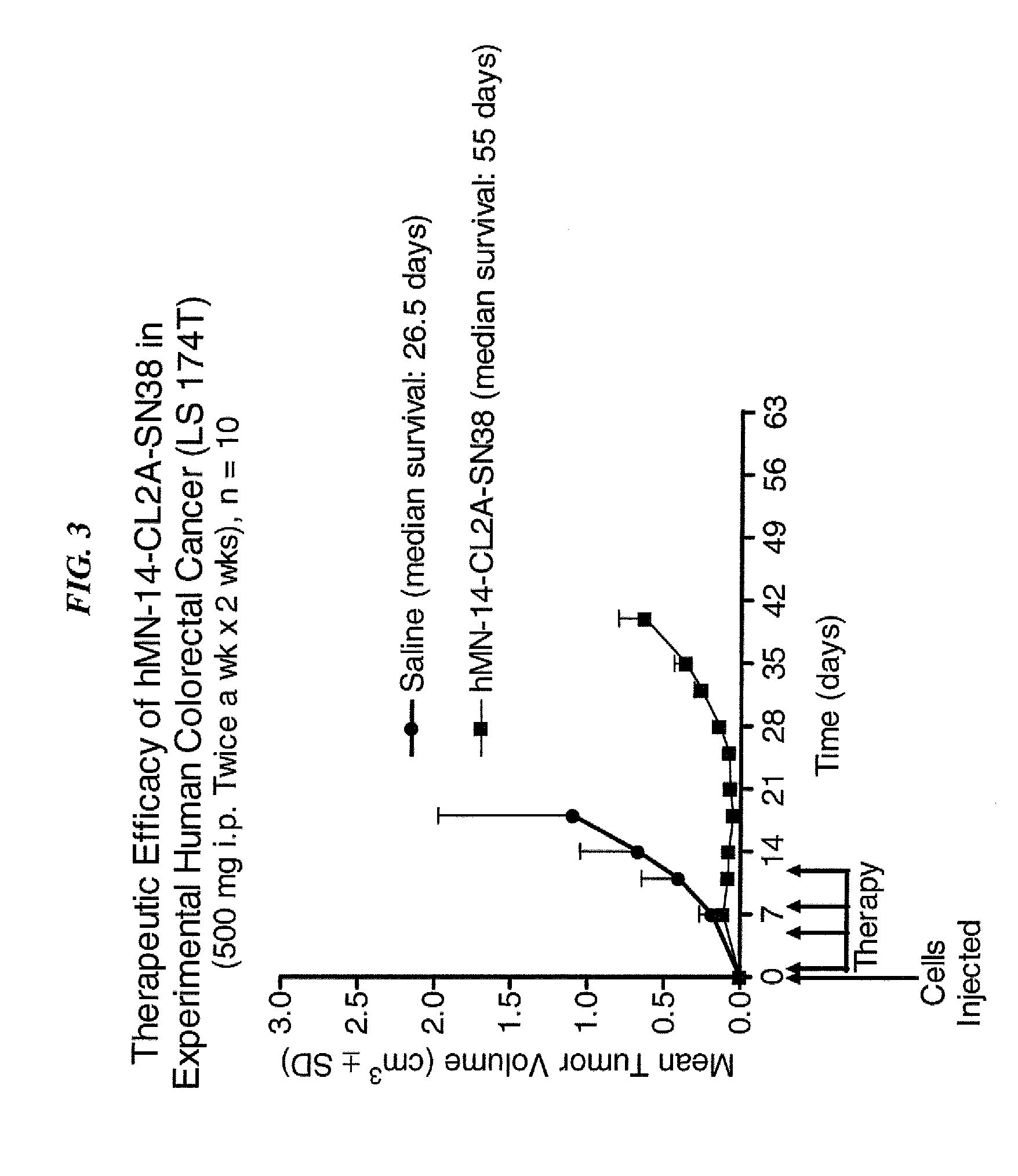
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140170063A1Overcome tumorImprove targetingHeavy metal active ingredientsOrganic active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 ROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140219914A1Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsRadioactive preparation carriersCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Mutated anti-cd22 antibodies with increased affinity to cd22-expressing leukemia cells
Recombinant immunotoxins are fusion proteins composed of the Fv domains of antibodies fused to bacterial or plant toxins. RFB4 (Fv)-PE38 is an immunotoxin that targets CD22 expressed on B cells and B cell malignancies. The present invention provides antibodies and antibody fragments that have improved ability to bind the CD22 antigen of B cells and B cell malignancies compared to RFB4. Immunotoxins made with the antibodies and antibody fragments of the invention have improved cytotoxicity to CD22-expressing cancer cells. Compositions that incorporate these antibodies into chimeric immunotoxin molecules that can be used in medicaments and methods for inhibiting the growth and proliferation of leukemia and lymphoma cells.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES
Modified antibodies against cd22 and utilization thereof
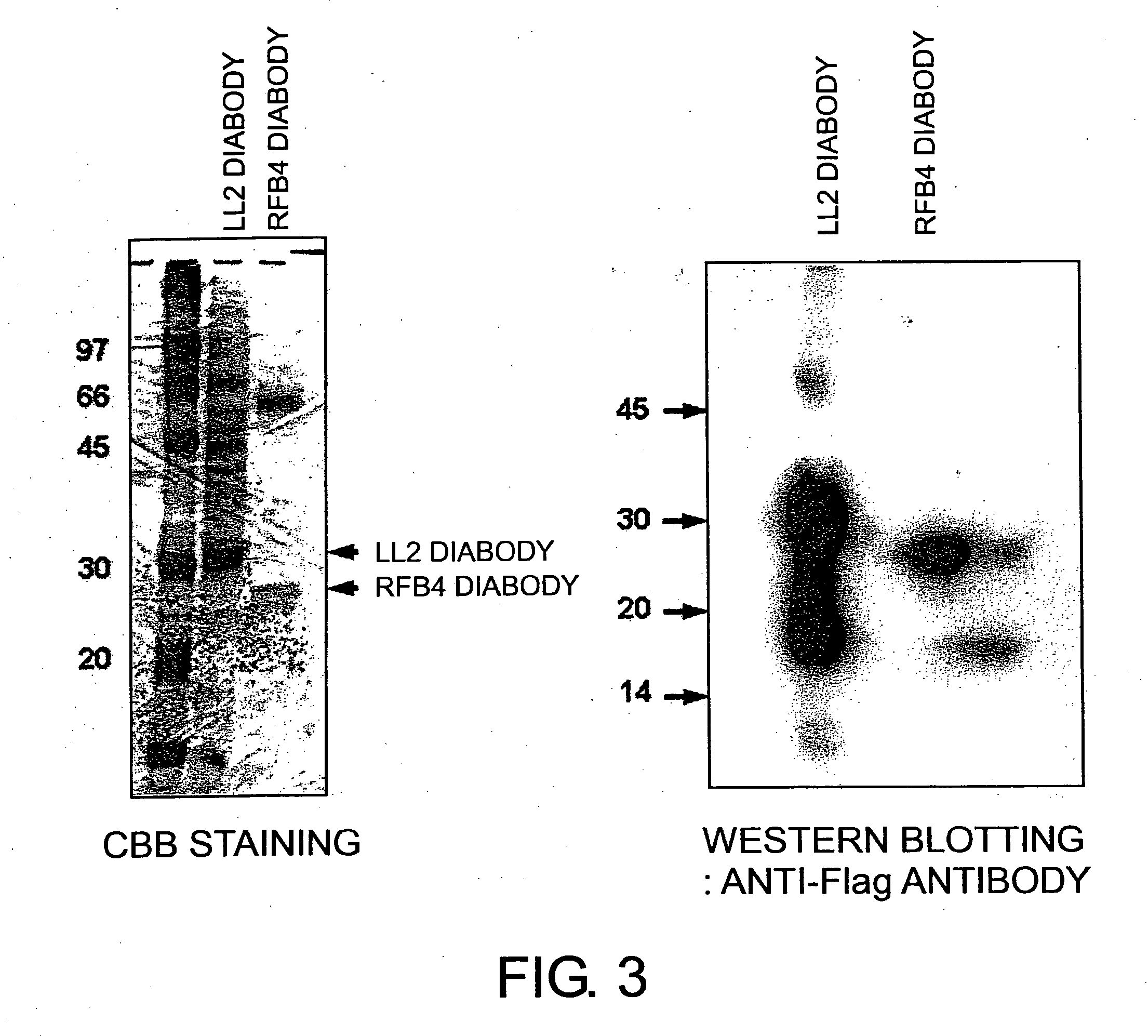
CD22 diabodies, in which heavy-chain and light-chain variable regions are linked by a 5-mer linker, were produced based on known sequence information for two types of anti-CD22 antibodies. The two diabodies produced were analyzed for their activity of binding to lymphoma cells and inducing lymphoma cell death (apoptosis). As a result, both diabodies were revealed to bind to the B-lymphoma cell line, “Raji”, and to have apoptosis-inducing activity towards Raji cells as well as towards another B-lymphoma cell line: Daudi cells. These results show that minibodies of antibodies that recognize CD22 can be used as apoptosis-inducing agents for tumor cells such as lymphoma cells.
Owner:CHUGAI PHARMA CO LTD
Dosages of immunoconjugates of antibodies and SN-38 for improved efficacy and decreased toxicity
ActiveUS9028833B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsHeavy metal active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
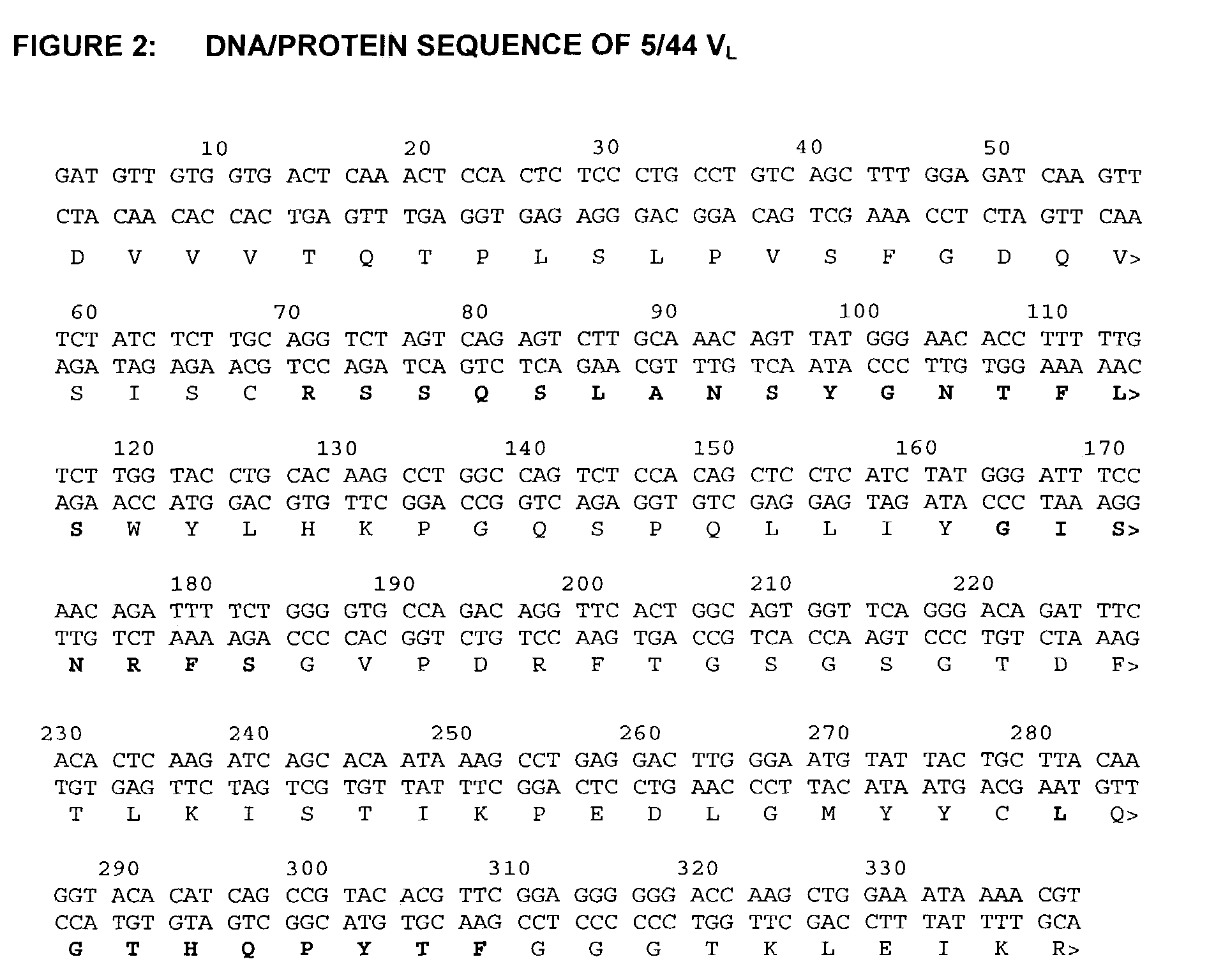
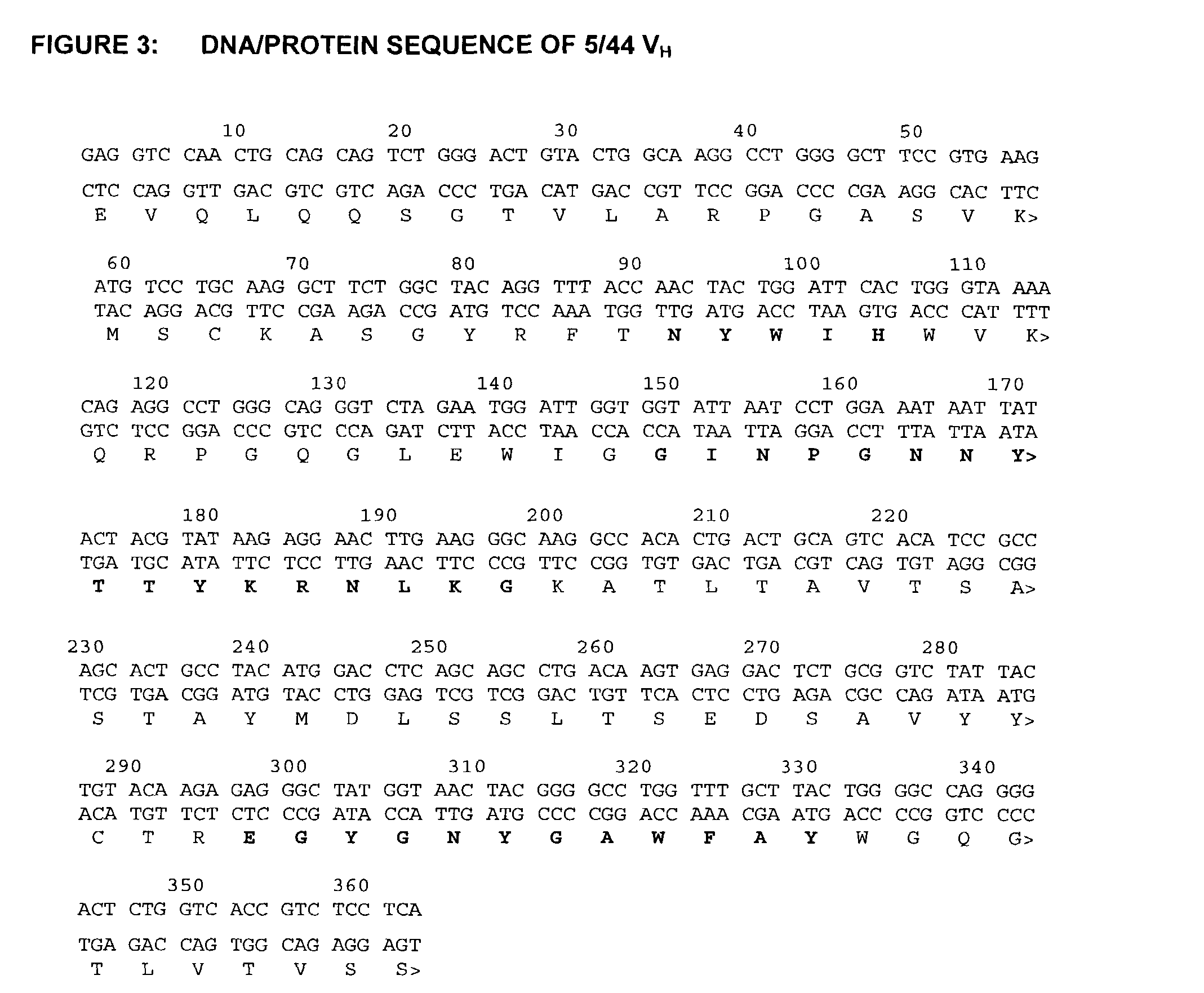
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS7829086B2Sugar derivativesImmunoglobulins against animals/humansAntigenImmunoglobulin Heavy Chain Variable Region
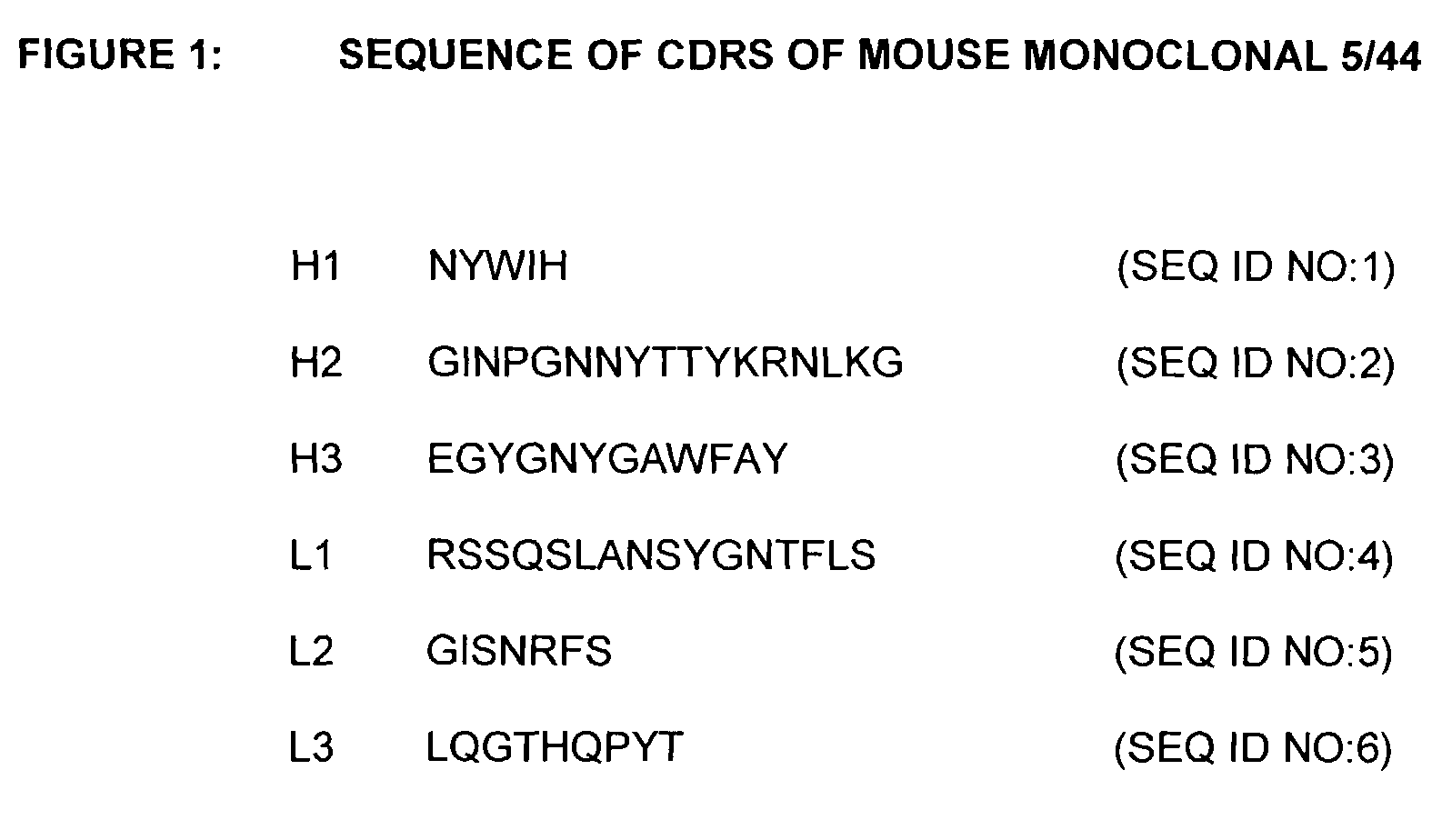
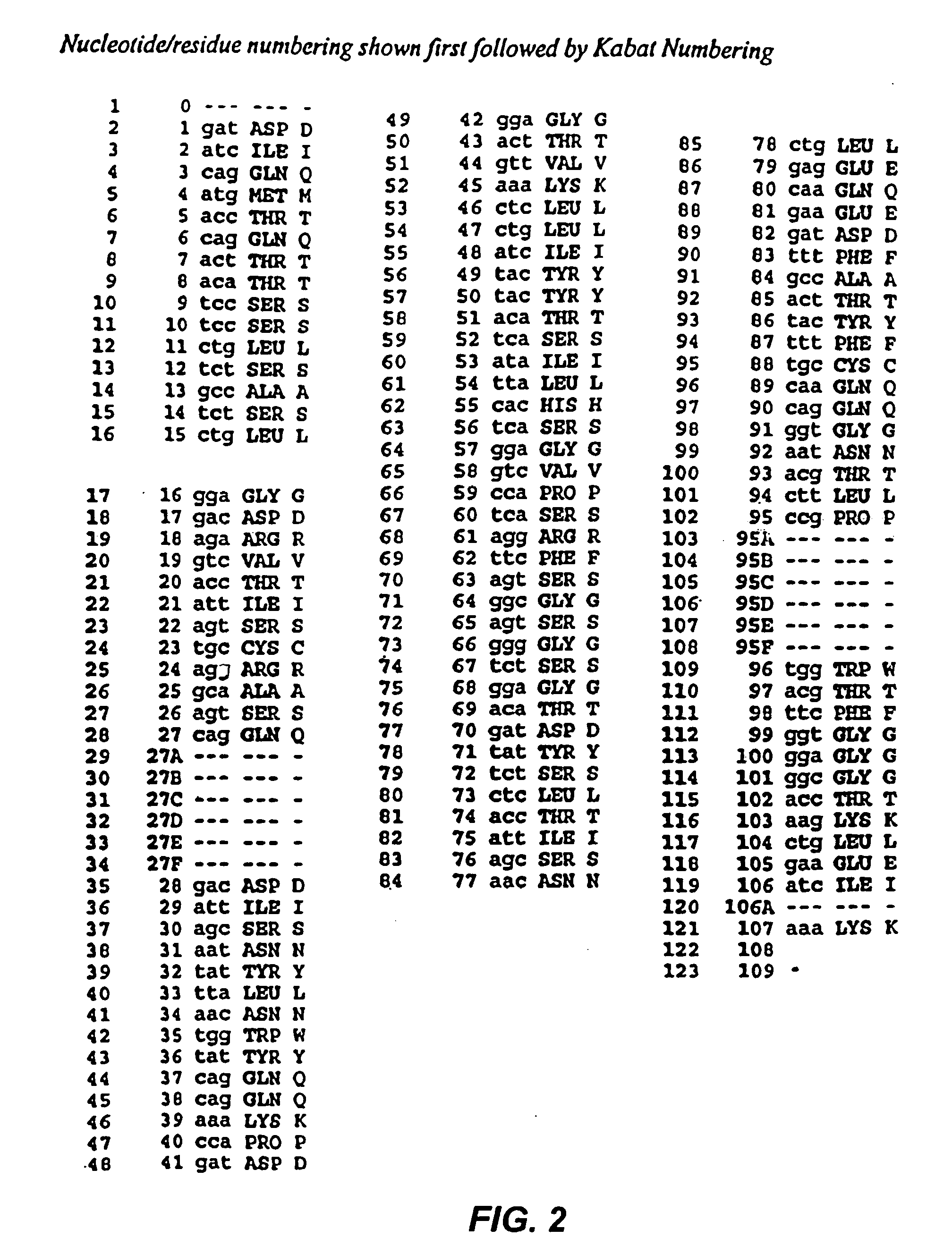
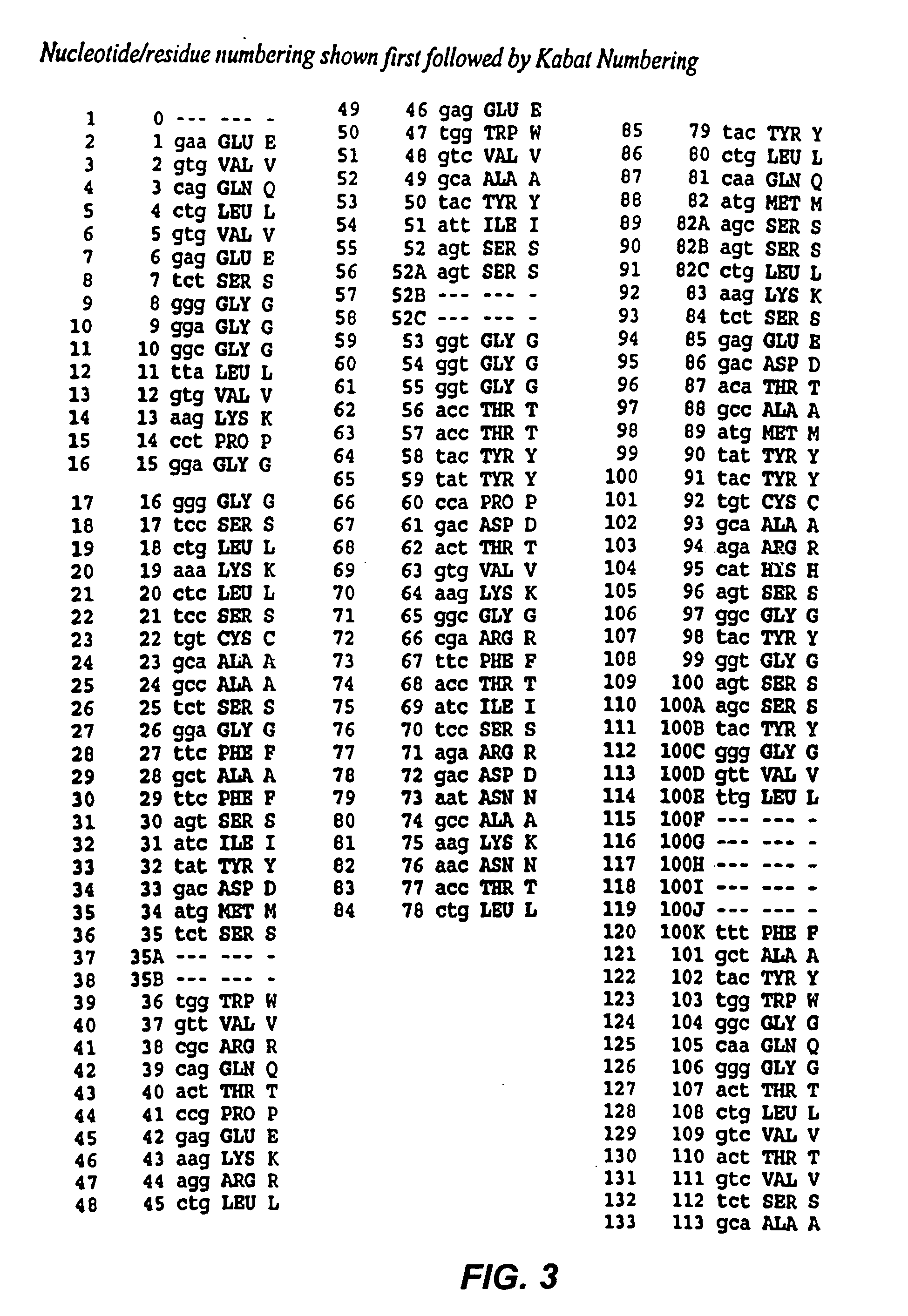
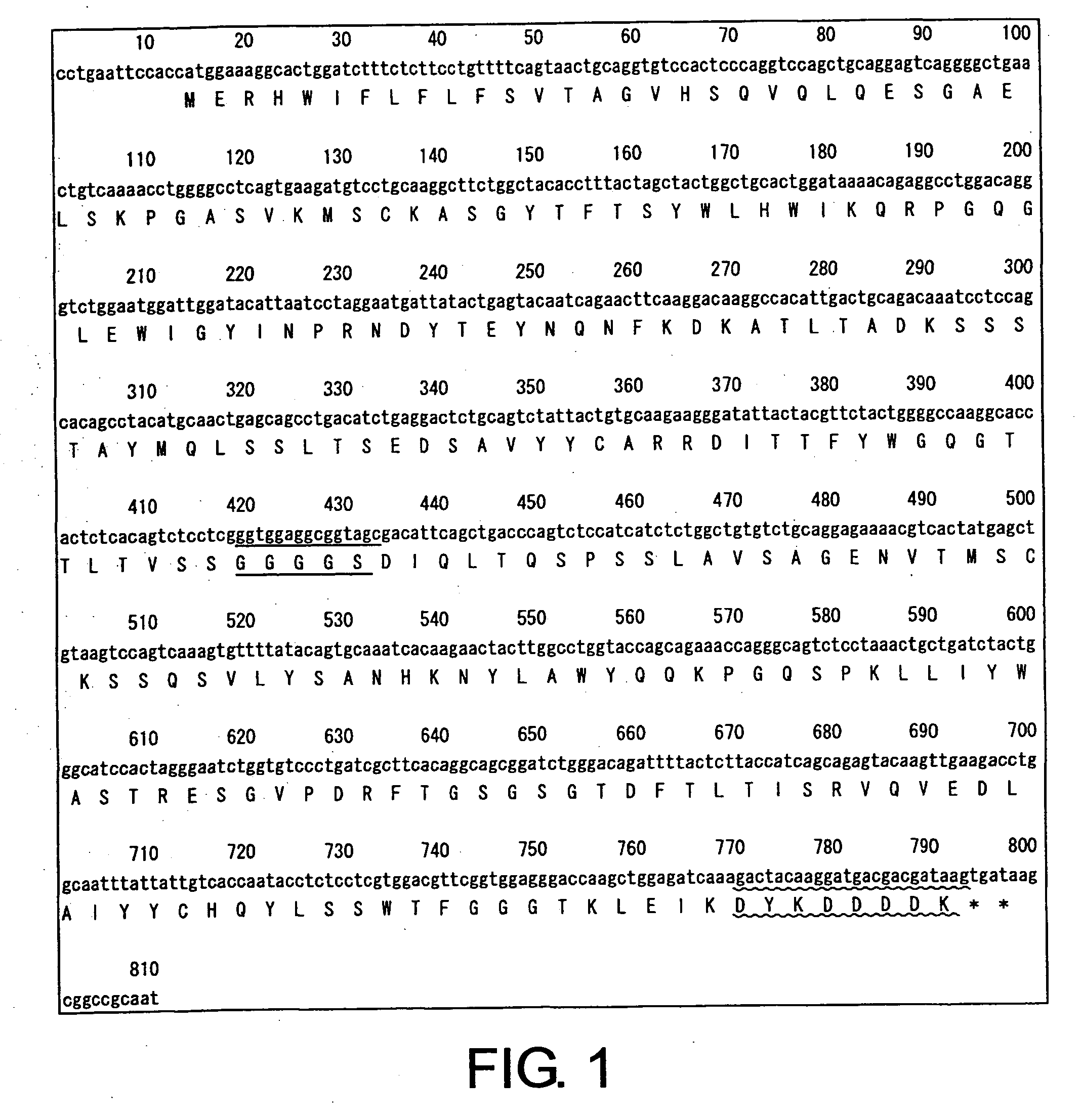
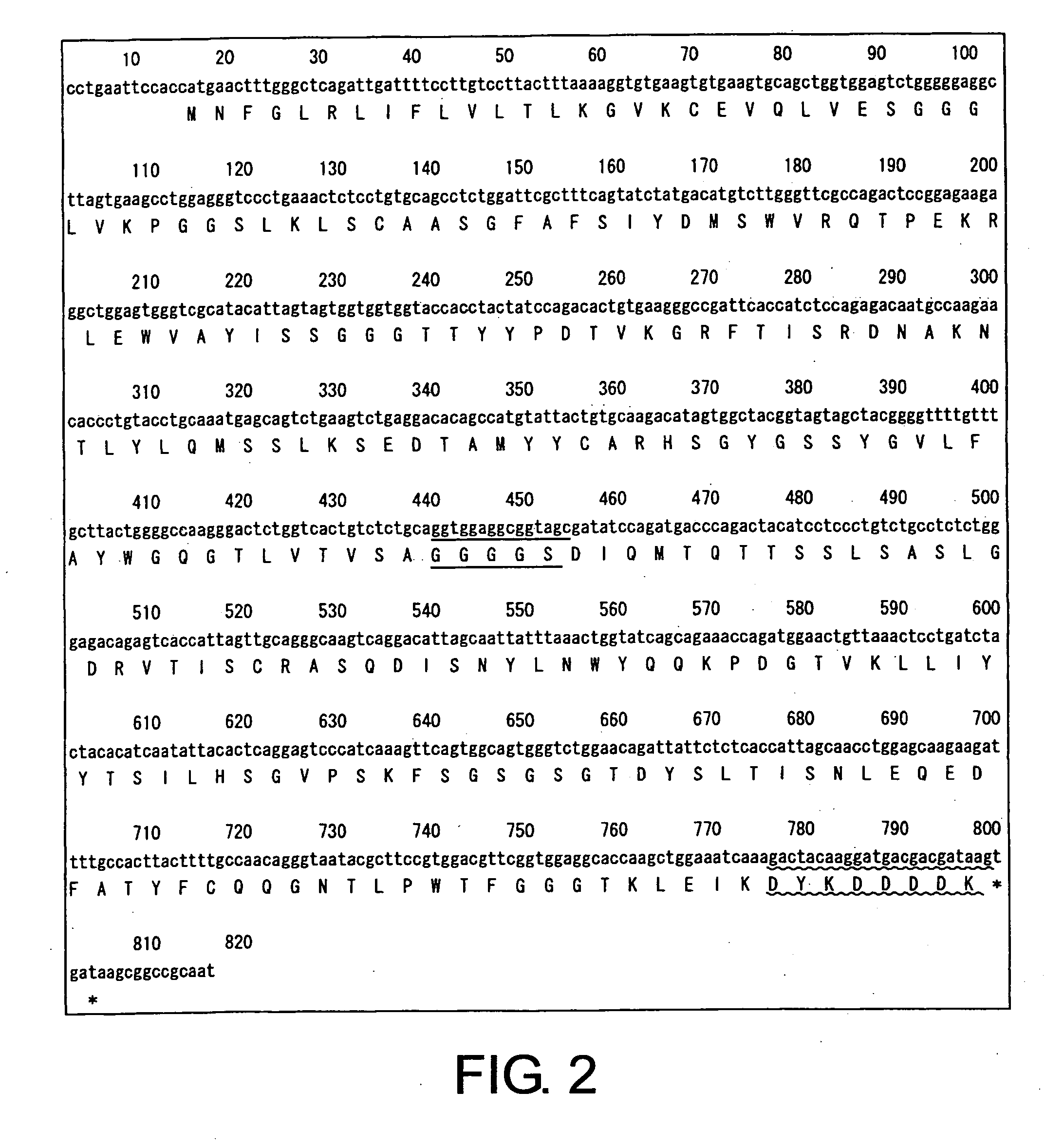
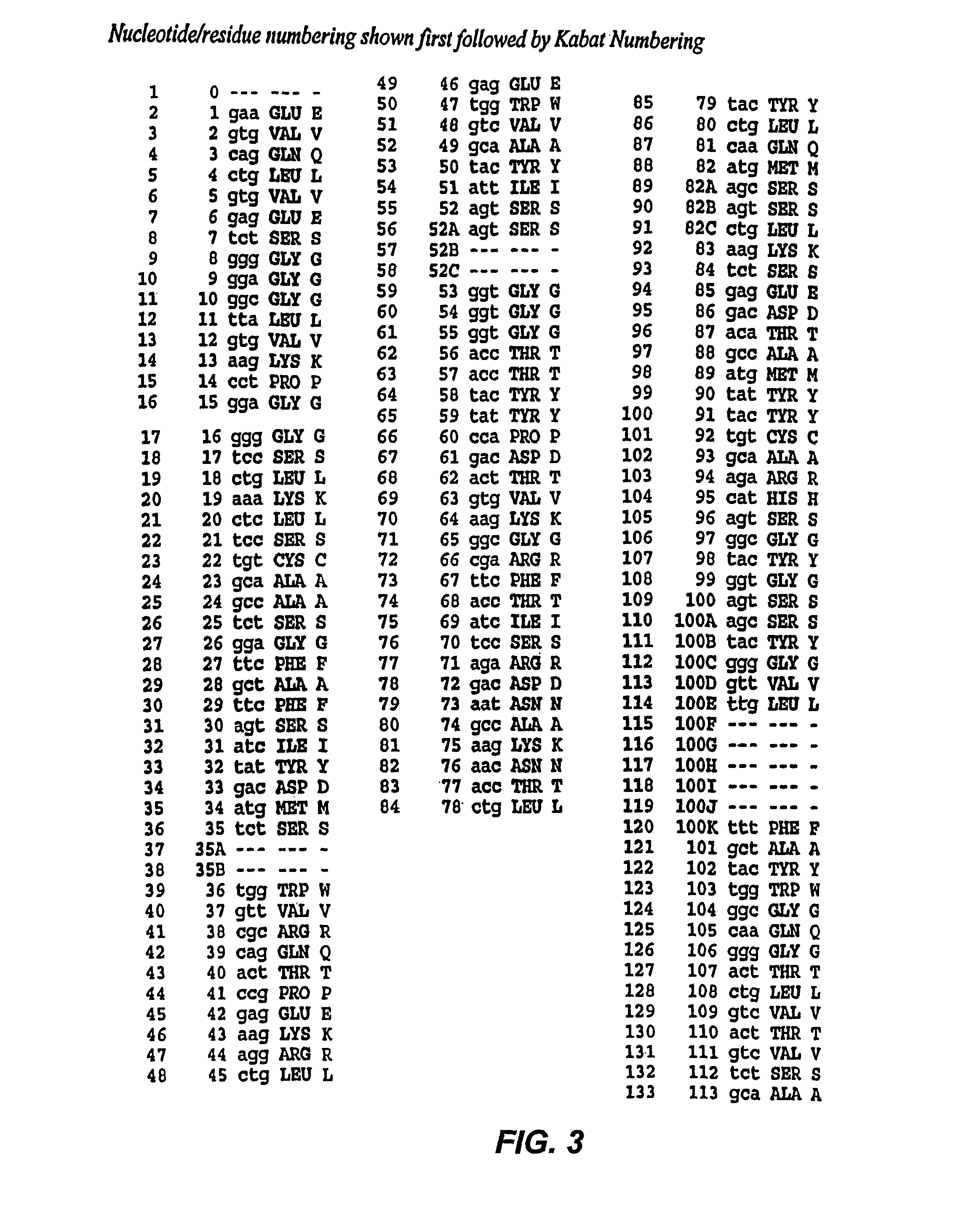
The present invention provides chimeric and humanized versions of anti-CD22 mouse monoclonal antibody, HB22.7. The anti-CD22 antibodies of the invention comprise four human or humanized framework regions of the immunoglobulin heavy chain variable region (“VH”) and four human or humanized framework regions of the immunoglobulin light chain variable region (“VK”). The invention further comprises heavy and / or light chain FW regions that contain one or more backmutations in which a human FW residue is exchanged for the corresponding residue present in the parental mouse heavy or light chain. Human or humanized VH framework regions of antibodies of the invention may comprise one or more of the following residues: a valine (V) at position 24 of framework region 1, a glycine (G) at position 49 of framework region 2, and an asparagine (N) at position 73 of framework region 3, numbered according to Kabat. The invention further relates to pharmaceutical compositions, immunotherapeutic compositions, and methods using therapeutic antibodies that bind to the human CD22 antigen and that preferably mediate human ADCC, CDC, and / or apoptosis for: the treatment of B cell diseases and disorders in human subjects, such as, but not limited to, B cell malignancies, for the treatment and prevention of autoimmune disease, and for the treatment and prevention of graft-versus-host disease (GVHD), humoral rejection, and post-transplantation lymphoproliferative disorder in human transplant recipients.
Owner:MEDIMMUNE LLC +1
Humanized anti-CD22 antibodies and their use in treatment of oncology, transplantation and autoimmune disease
InactiveUS20070258981A1Efficient productionEfficiently depletedSugar derivativesImmunoglobulins against animals/humansAntigenAutoimmune condition
Owner:MEDIMMUNE LLC +1
Synergistic anti-CD47 therapy for hematologic cancers
Methods are provided for treatment of hematologic cancers, particularly lymphomas and leukemias, including without limitation myelogenous and lymphocytic leukemias. A combination of antibodies specific for CD47; and specific for a cancer associated cell surface marker are administered to the patient, and provide for a synergistic decrease in cancer cell burden. The combination of antibodies may comprise a plurality of monospecific antibodies, or a bispecific or multispecific antibody. Markers of interest include without limitation, CD20, CD22, CD52, CD33; CD96; CD44; CD123; CD97; CD99; PTHR2; and HAVCR2.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Mutated anti-cd22 antibodies and immunoconjugates
ActiveUS7982011B2Immunoglobulin superfamilyAntibody mimetics/scaffoldsAntiendomysial antibodiesCancer cell
Recombinant immunotoxins are fusion proteins composed of the Fv domains of antibodies fused to bacterial or plant toxins. RFB4 (Fv)-PE38 is an immunotoxin that targets CD22 expressed on B cells and B cell malignancies. The present invention provides antibodies and antibody fragments that have improved ability to bind the CD22 antigen compared to RFB4. Immunotoxins made with the antibodies and antibody fragments of the invention have improved cytotoxicity to CD22-expressing cancer cells. Compositions that incorporate these antibodies into chimeric immunotoxin molecules that can be used in medicaments and methods for inhibiting the growth and proliferation of such cancers. Additionally, the invention provides a method of increasing the cytotoxicity of forms of Pseudomonas exotoxin A (“PE”) with the mutation of a single amino acid, as well as compositions of such mutated PEs, nucleic acids encoding them, and methods for using the mutated PEs.
Owner:UNITED STATES OF AMERICA
Pyrrolobenzodiazepine-Anti-cd22 antibody conjugates
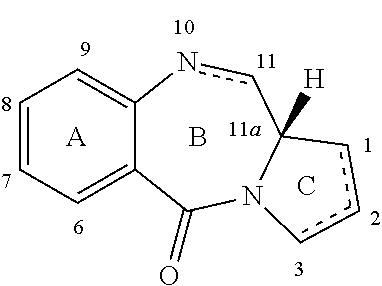
ActiveUS20150265722A1Aid solubilityIncrease the number ofOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody conjugatePyrrolobenzodiazepine
Owner:MEDIMMUNE LTD +1
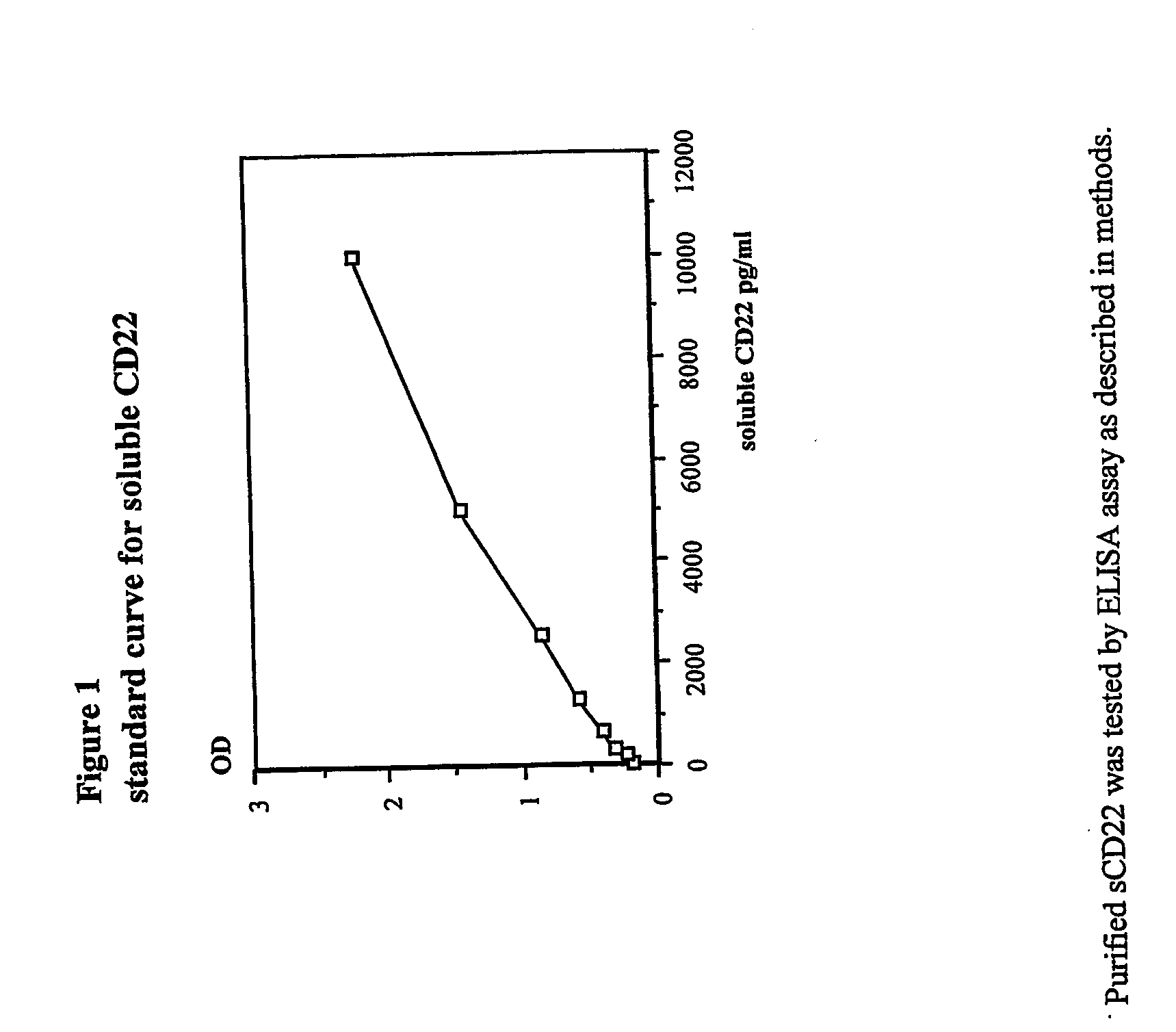
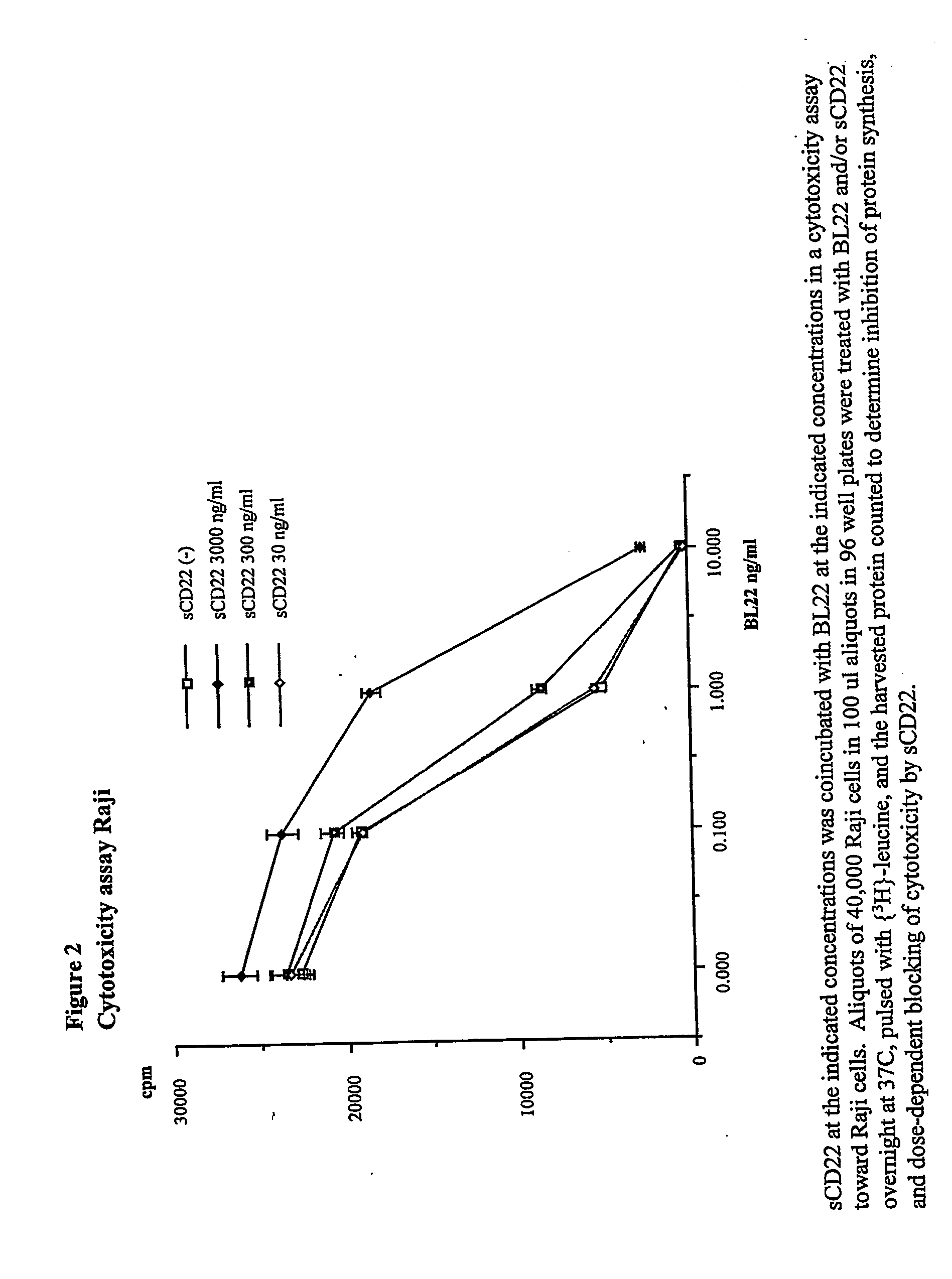
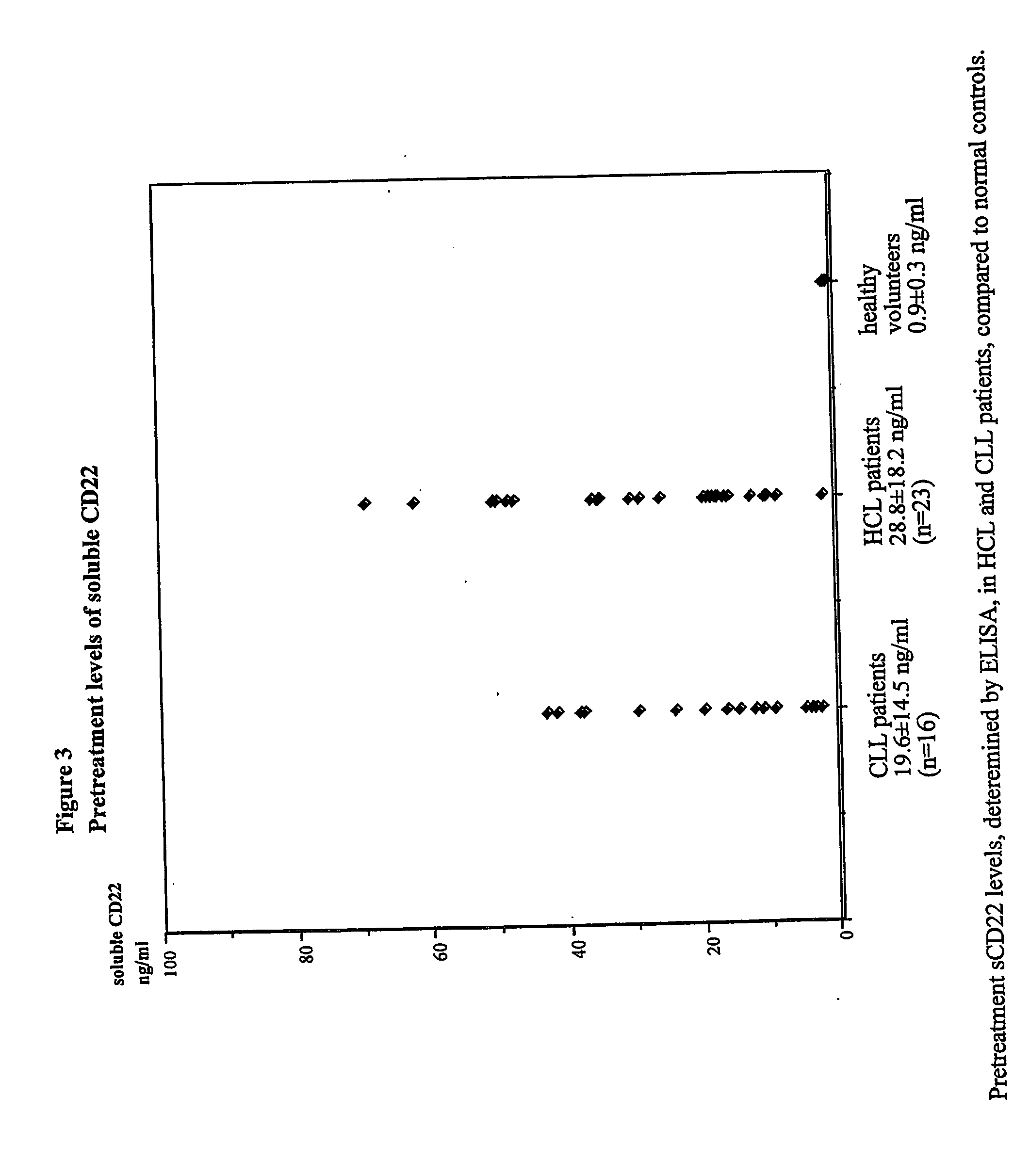
Elisa assay of serum soluble cd22 to assess tumor burnden/relapse in subjects with leukemia and lymphoma
InactiveUS20050244828A1Microbiological testing/measurementBiological material analysisAbnormal tissue growthTumor Load
Disclosed herein are methods of using previously unknown soluble forms of CD22 (sCD22) present in the serum of subjects with B-cell leukemias and lymphomas to assess tumor burden in the subjects. Also disclosed are methods of diagnosing or prognosing development or progression of a B-cell lymphoma or leukemia in a subject, including detecting sCD22 in a body fluid sample taken or derived from the subject, for instance serum.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICAS AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES THE
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20050180975A1Avoiding and decreasing and resistanceIncrease ratingsBiocidePeptide/protein ingredientsBiological activationHematologic malignancy
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN®.
Owner:BIOGEN INC
Immunoconjugates with improved efficacy for the treatment of diseases
InactiveUS20070196274A1Improve therapeutic efficacyHigh detection sensitivityIn-vivo radioactive preparationsImmunoglobulins against animals/humansAntibody conjugateCD11a
The invention provides therapeutic or diagnostic antibodies with modified N— or C-terminal sequences that are enriched with lysine or tyrosine residues. These lysine or tyfosine residues can be used to couple radioisotopes, cytotoxic agents, or detectable labels. The increased stoichiometric ratios of these agents in the antibody conjugates lead to improved therapeutic efficacy or enhanced detection sensitivity. Non-limiting examples of antibodies suitable for the present invention include anti-CD22, anti-ErbB2, anti-VEGF, anti-EGFR, anti-VEGFR, anti-Her-3, anti-Her-4, anti-CEA, anti-CTLA-4, anti-CD4, anti-CD3, anti-CD20, anti-TNF-a, anti-CD11a, anti-Lewis Y antigen, anti-TrailR, anti-IL2R, anti-CD30, anti-CD146, anti-CD147, anti-alpha V integrin beta, anti-CD19, anti-GD2, anti-3H11, anti-EBV, anti-HIV, anti-HBV, anti-HCV, and other disease-specific antibodies.
Owner:WELSON PHARMA
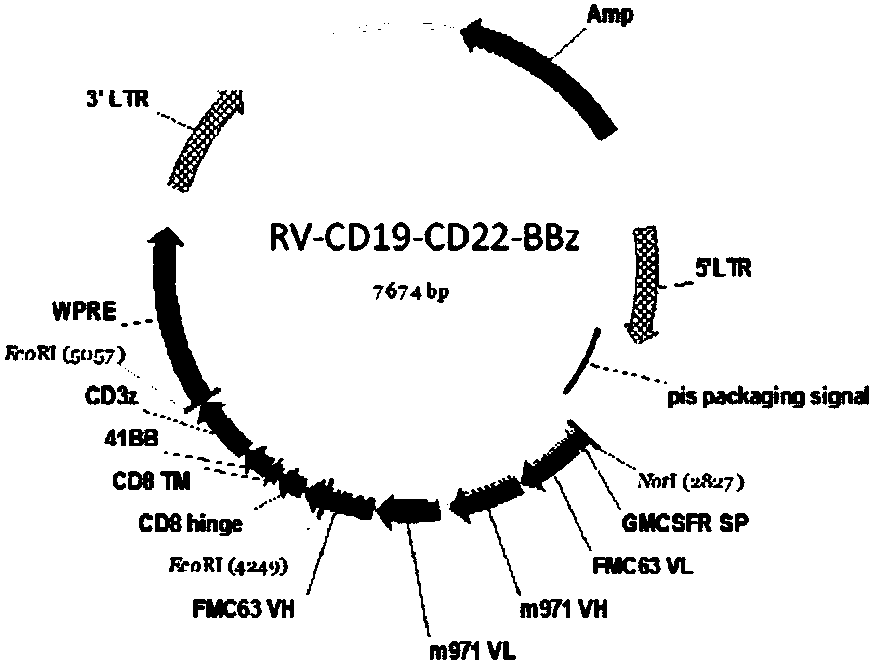
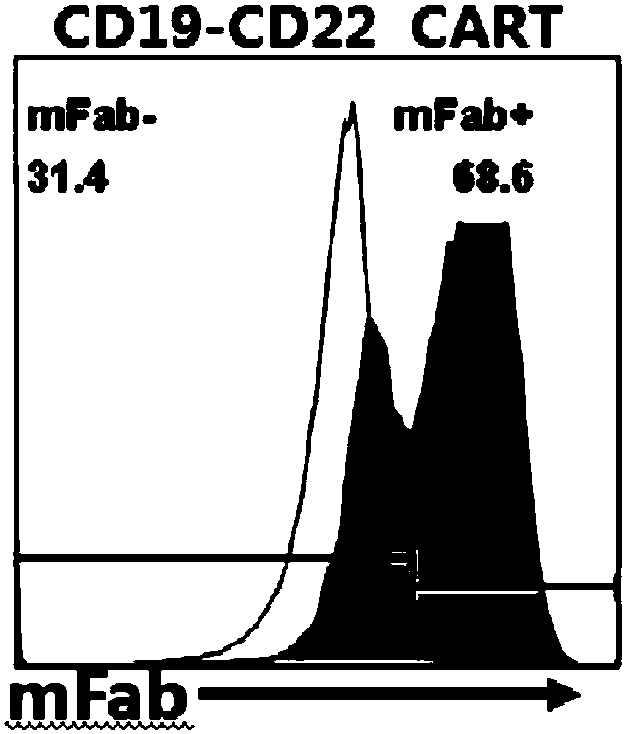
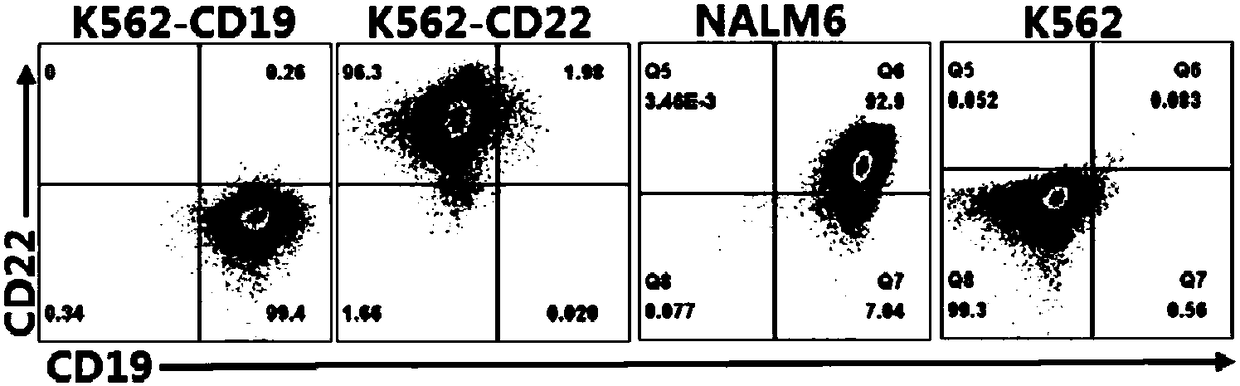
Target CD19 and CD22 chimeric antigen receptor and application thereof
PendingCN108504668AShorten the timeStable expressionMammal material medical ingredientsImmunoglobulinsSingle-Chain AntibodiesAntigen receptors
The invention relates to a double-target CD19 and CD22 chimeric antigen receptor and application thereof, in particular to a polynucleotide sequence which is selected from (1) a coding sequence comprising anti-CD22 and anti-CD19 single-chain antibodies which are connected sequentially, a coding sequence of a human CD8 hinge region, a coding sequence of human CD8 transmembrane region, a coding sequence of a human 41BB intracellular region, a coding sequence of a human CD3 zeta intracellular region and a polynucleotide sequence of a coding sequence of a fragment, comprising an extracellular domain III and a extracellular domain IV, of an optional EGFR; and (2) a complementary sequence of the (1) polynucleotide sequence. The invention further provides a relevant fusion protein, a carrier comprising the coding sequence and application of the fusion protein, the coding sequence and the carrier. The prepared CD19-CD22-BBzCAR-T cell has a strong killing function to the specific tumor cell, CD107a expression and IFN gamma secretion are high, and the killing efficiency to the target cell can reach about 80% under the situation that the effector-target ratio is 10:1.
Owner:HRAIN BIOTECHNOLOGY CO LTD
Reagents and treatment methods for autoimmune diseases
InactiveUS20080118505A1Clinical improvementImprove curing speedSenses disorderAntipyreticDiseaseEpitope
The invention concerns treatment methods using anti-CD22 monoclonal antibodies with unique physiologic properties. In particular, the invention concerns methods for the treatment of B-cell malignancies and autoimmune diseases by administering an effective amount of a blocking anti-CD22 monoclonal antibody specifically binding to the first two Ig-like domains, or to an epitope within the first two Ig-like domains of native human CD22 (hCD22).
Owner:DUKE UNIV
Immunoregulatory antibodies and uses thereof
InactiveUS20080227198A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com