Reagents and treatment methods for autoimmune diseases
a technology of autoimmune diseases and antibodies, applied in the field of anti-anticd22 monoclonal antibodies, can solve problems such as significant morbidity and disability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Anti-CD22 Monoclonal Antibodies
[0122]Monoclonal antibodies (mAbs) HB22-7 (IgG2b), HB22-23 (IgG2a) 1HB22-33 (IgM), HB22-5 (IgG2a), HB22-13 (IgG2a), HB22-22 (IgA), and HB22-196 were produced according to the method of Engel et al., J Immunol 15:4710 (1993) and U.S. Pat. No. 5,484,892. See, also Tuscano et al, Blood 94:1382-1392 (1999). However, other methods may be used. Briefly, the HB22 mAbs were produced via hybridoma techniques using a mouse pre-B cell line 300.19, stably transfected with full length CD22 cDNA, as the immunogen. More specifically, thirty-three mAbs reactive with CD22 were generated by the fusion of NS-1 myeloma cell with spleen cells from Balb / c mice immunized three times with a mouse pre-B cell line, 300.19, stably transfected with a full-length CD22 cDNA. Hybridomas producing mAb reactive with mouse L cells transfected with CD22 cDNA, but not with untransfected cells, were cloned twice and used to generate supernatant or ascites fluid. mAb isotypes...
example 2
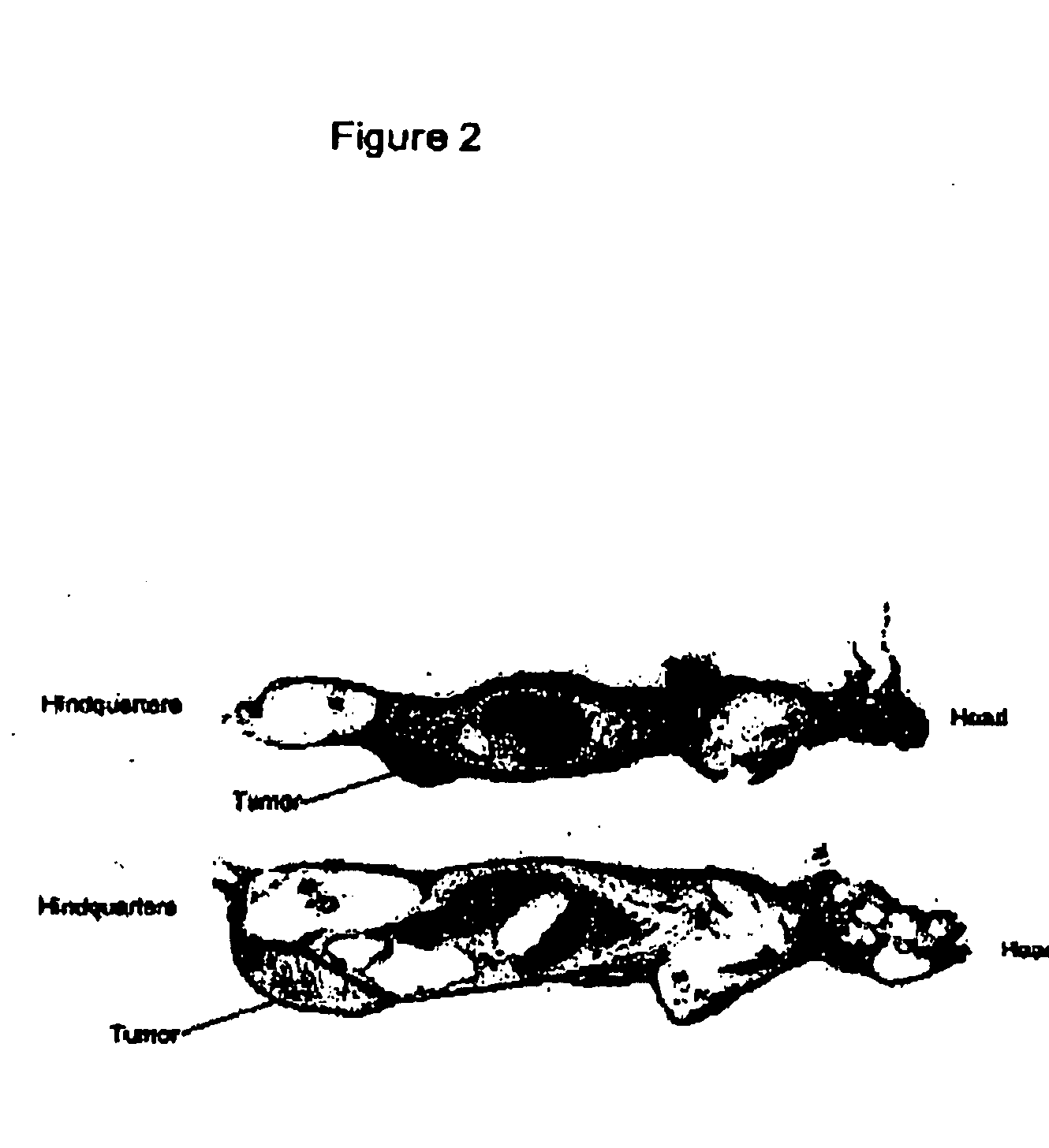
Raji and Ramos Lymphoma Xenograft Trials
[0124]This example describes the results from our independent Raji and Ramos lymphoma xenograft trials. Nude mice xenografts are important tools for preclinical evaluations. Nude mice bearing human non-Hodgkin's lymphoma (NHL) xenografts utilizing the lymphoma cell lines Raji and Ramos have proven utility for evaluating efficacy for treatment of NHL. (Buchsbaum et al., Cancer Res. 52(23):6476-6481 (1992) and Flavell et al., Cancer Res. 57:4824-4829 (1997)).
[0125]Materials and Methods
[0126]Reagents. Carrier-free 90Y (Pacific Northwest National Laboratory, Richland, Wash.) and 111In (Nordion, Kanata, Ontario, Canada) were purchased as chlorides in dilute HCl. Lym-1 (Techniclone, Inc Tustin, Calif.) is an IgG2a mAb generated in mice immunized with human Burkitt's lymphoma cell nuclei. Lym-1 recognizes a cell surface 31-35 kD antigen on malignant B cells, and reacts with greater than 80% of human B cell NHL. Lym-1 purity was assessed according to ...
example 3
Sequence Analysis of Anti-CD22 Antibodies
[0156]VH and Light Chain Gene Utilization
[0157]Cytoplasmic RNA was extracted from 1−10×105 hybridoma cells using the RNeasy Mini Kit (Qiagen Chatsworth, Calif.). First strand cDNA was synthesized from cytoplasmic RNA using oligo-dT primers (dT18) and a Superscript Kit (Gibco BRL, Gaithersburg, Md.). One μl of cDNA solution was used as template for PCR amplification of VH genes. PCR reactions were carried out in a 100-μl volume of a reaction mixture composed of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 μM dNTP (Perkin Elmer, Foster City, Calif.), 50 μmol of each primer, and 5 U of Taq polymerase (ISC Bioexpress, Kaysville, Utah). Amplification was for 30 cycles (94° C. for 1 min, 58° for 1 min, 72° C. for 1 min; Thermocycler, Perkin Elmer). VH genes were amplified using a promiscuous sense 5′ VH primer (Ms VHE: 5′ GGG AAT TCG AGG TGC AGC TGC AGG AGT CTG G 3′; SEQ ID NO: 2) as previously described (Kantor et al., J. Immunol. 158:117...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com