Proinsulin peptides for type 1 diabetes
A type 1 diabetes and disease technology, applied in the direction of insulin, hormone peptides, peptides, etc., can solve the problems of not being disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Materials and methods
[0088] animal
[0089] Make HLA-DR3-DQ2-transgenic animals (B6.hCD4.DR3-DQ2.DQ2.MHCII - / - Mice (described in the past (de Kauwe AL et al. (2009) J Immunol 182 (12): 7440-7450) and obtained from J. McCluskey) with RIP-B7.1 transgenic animals (B6.Cg- Tg(Ins2-CD80)3B7Flv / Orl, EMMA, Orleans, France; ID00216) hybridization to obtain HLA-DR3-DQ2 + huCD4 + IA / IE - / - RIP.B7.1 + mouse, hereinafter referred to as DR3DQ2xRIP-B7.1. By making B6.129S2-H2-Ab1 tm1Gru DR4xRIP-B7.1 mice produced by crossing Tg(HLA-DRA / H2-Ea,HLA-DRB1*0401 / H2-Eb)1Kito mice with B6.Cg-Tg(Ins2-CD80)3B7Flv / Orl have been used in the past described (Verhagen J, et al. (2018) Sci Rep 8(1):14106). All animals were housed under specific pathogen-free conditions in individually ventilated cages in the KCL Biological Services Unit on a 12-h light / dark cycle with food and water provided ad libitum. Experiments were carried out under a project license held by M.Peakman in accordanc...
Embodiment 2
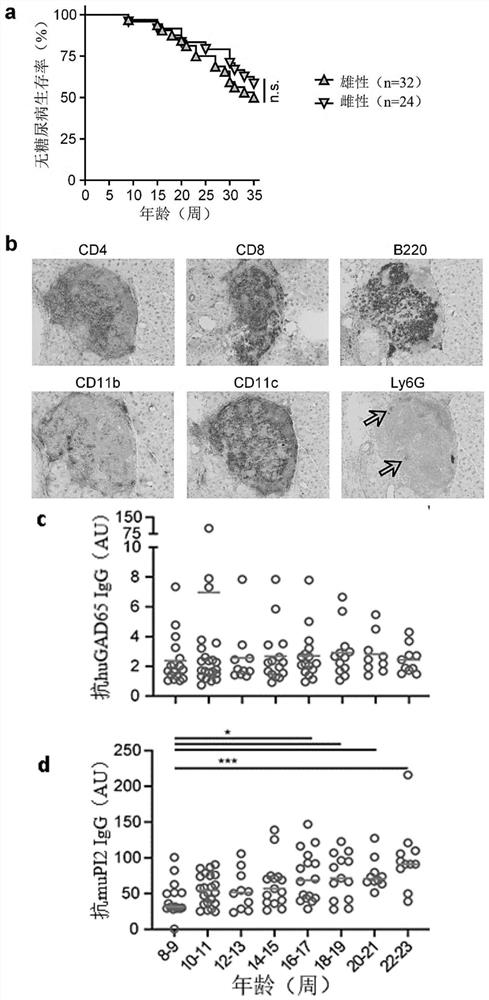
[0110] Spontaneous diabetes onset in DR3DQ2xRIP-B7.1 mice
[0111] Unlike our previously described DR4xRIP-B7.1 model (VerhagenJ, et al. (2018) Sci Rep 8(1):14106), which did not display spontaneous insulitis or diabetes, DR3DQ2xRIP-B7.1 mice spontaneously developed diabetes ( figure 1 a). Although both models exhibited relatively high baseline levels of blood glucose at any age, only DR3DQ2xRIP-B7.1 mice developed levels >16.7 mmol / l in addition to glycosuria. By 35 weeks of age, 46% (26 of 56) of all animals monitored had developed autoimmune diabetes. Disease incidence (16 / 32 vs. 10 / 24, respectively) and mean age of onset (24.2 weeks ± 7.3 (SD) vs. 24.8 ± 8.5, respectively) were similar in male and female animals. No overt signs of other immune-mediated disorders were detected, as evidenced by splenomegaly, cachexia, lethargy, or skin / ocular abnormalities. All mice with diabetes showed severe immune infiltration in islets. This infiltrate is highly diverse, with both...
Embodiment 3
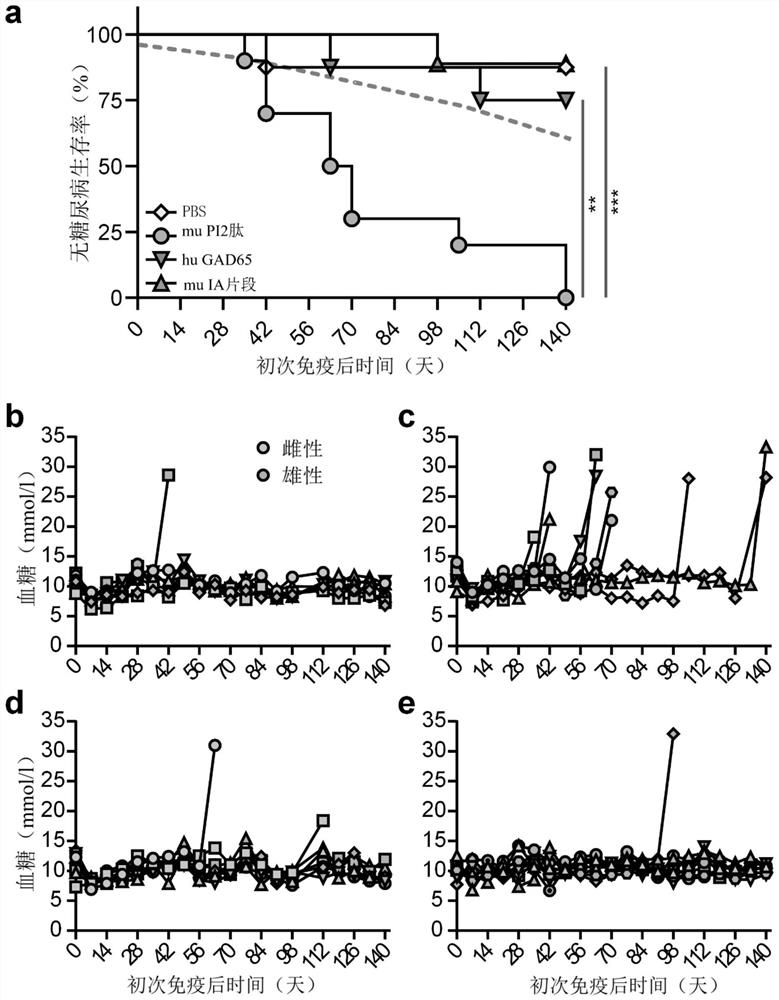
[0113] Proinsulin is a diabetogenic antigen in DR3DQ2xRIP-B7.1 mice
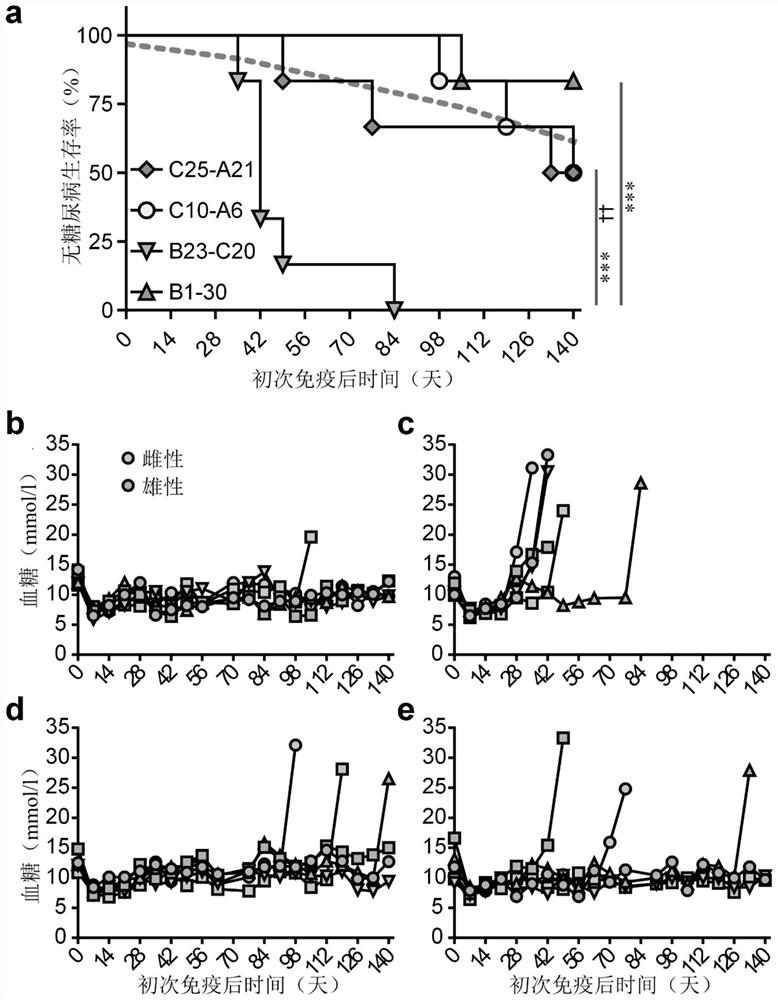
[0114] Spontaneous diabetes onset in mice carrying high-risk transgenic HLA suggests that autoimmune processes are central to disease development and prompted us to address the question of whether specific autoantigens are disease drivers. The hypothesis that priming an animal with an adjuvant against a candidate molecule will accelerate disease progression has been tested if the antigen has 'driver' properties. Thus, single 30-mer peptides overlapping and spanning the length of murine proinsulin 2 in TiterMax Gold adjuvant; recombinant human GAD65; the 377 amino acid intracellular region of human islet antigen-2 (IA-2) or individual Mice were first immunized with PBS ( figure 2 a-f)( figure 2 a-f). Importantly, in these conditions, primary immunization with proinsulin peptide alone significantly accelerated the onset of diabetes and increased morbidity beyond that observed with control stimulation or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com