Blood-based tumor mutation burden (bTMB) biomarker, and measuring method and uses thereof
A biomarker and sequencing technology, applied in the determination/test of microorganisms, biochemical equipment and methods, etc., can solve problems such as the inability to effectively predict the OS benefit of tumor patients receiving immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
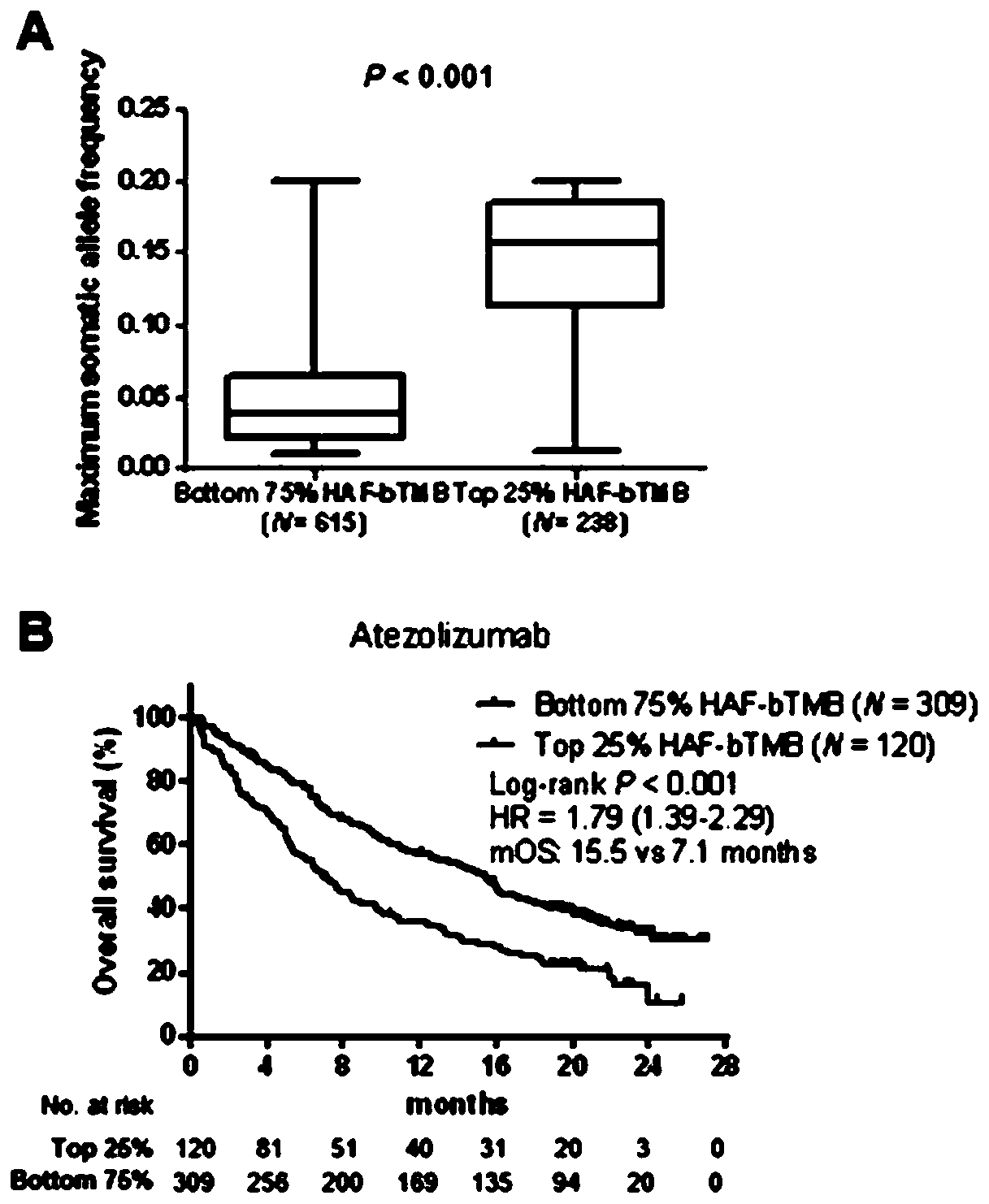
[0052] Immunotherapy versus chemotherapy in patients with non-small cell lung cancer, and within the immunotherapy group, the prognosis for OS and PFS benefit Measurement
[0053] Sources of bTMB detection data and patient clinicopathological data
[0054] Firstly, data from POPLAR (NCT01903993, N=211) and OAK (NCT02008227, N=642) studies were combined. The POPLAR study is a phase II randomized controlled trial comparing second / third-line atezolizumab with standard-of-care docetaxel chemotherapy in patients with advanced or metastatic non-small cell lung cancer who were not screened for PD-L1 expression. The OAK study was a phase III randomized controlled trial comparing atezolizumab versus docetaxel chemotherapy in patients with metastatic non-small cell lung cancer. bTMB detection data and patient clinicopathological parameters in both studies were white https: / / clinicalstudvdatarecluest.com / .
[0055] LAF-bTMB Calculation
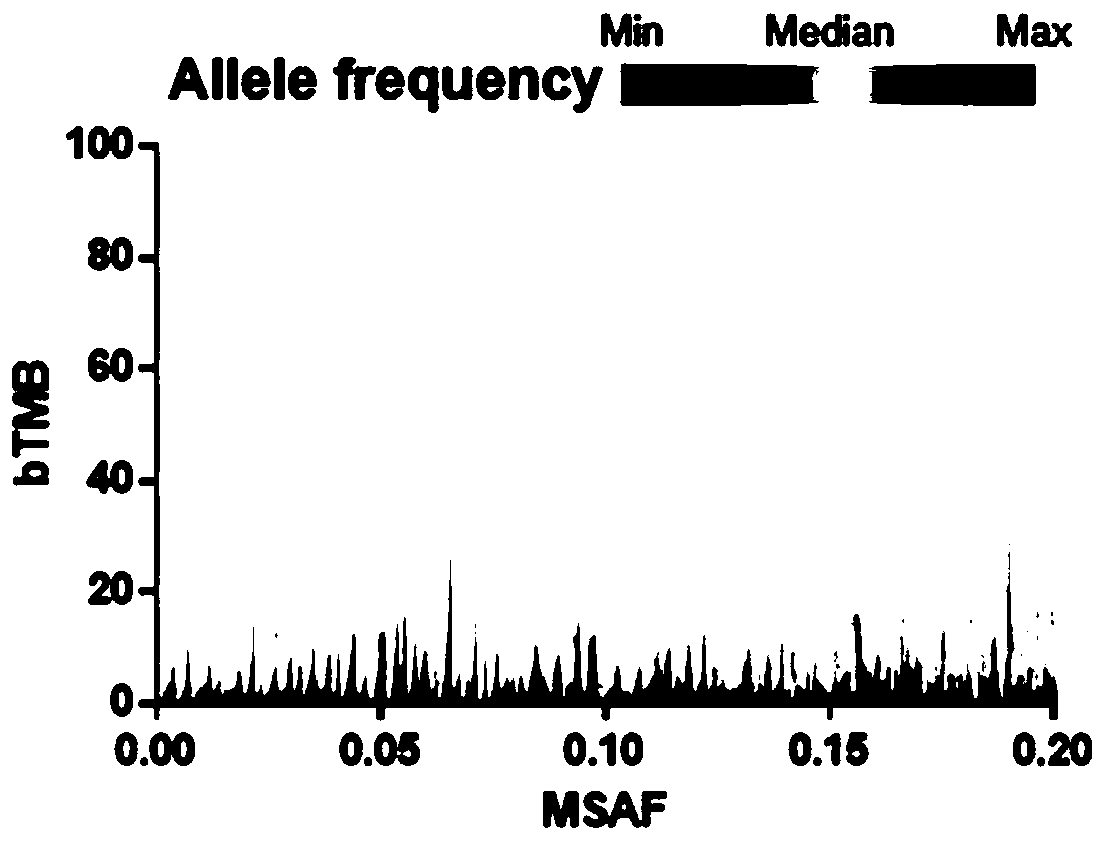
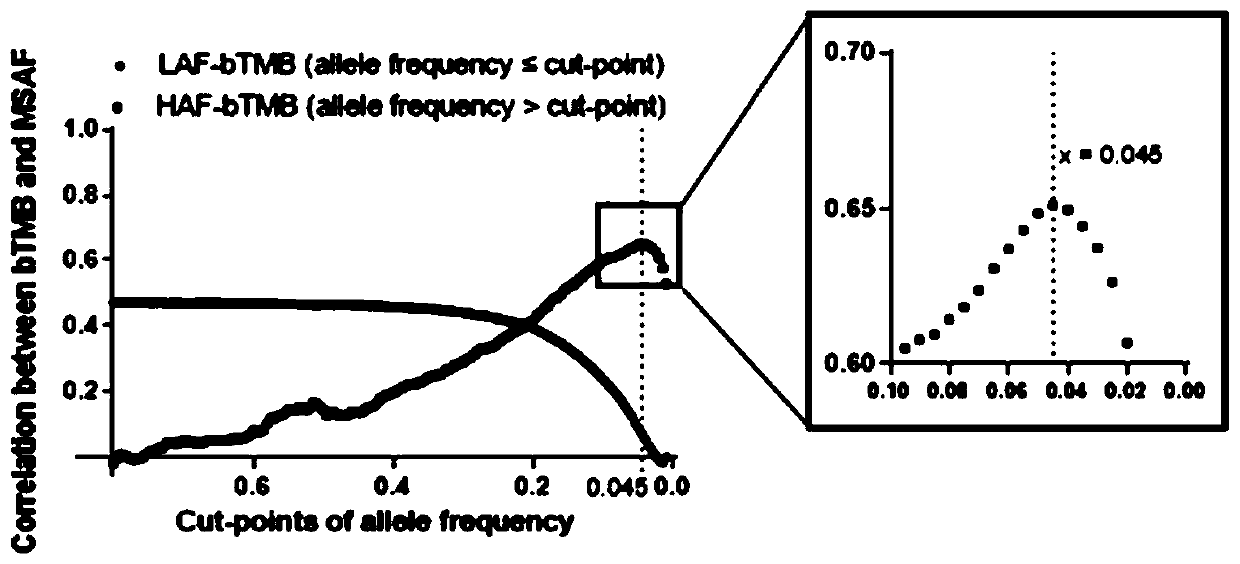
[0056] The sum of the number of s...
Embodiment 2
[0072] LAF-bTMB guides immunotherapy for non-small cell lung cancer patients in China
[0073] Patient Recruitment
[0074] From August 1, 2016 to January 1, 2018, 64 patients with advanced non-cancerous patients receiving first / second / third-line immunotherapy (anti-PD-1 / PD-L1) were enrolled in Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College Hospital. patients with small cell lung cancer. This study was approved by the ethics committees of all participating institutions. All enrolled patients signed an informed consent form before the start of the study. The recruited patients are hereinafter referred to as the NCC cohort.
[0075] bTMB detection and LAF-bTMB calculation
[0076] The detection method of bTMB has been disclosed in the document JAMA Oncol.2019Feb 28.doi:10.1001 / jamaoncol.2018.7098. The panel used for the detection is NCC-GP150 covering 150 genes, which has also been disclosed in the document JAMAOncol.2019Feb 28...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com