T cell synergist for CAR T cell therapy of leukemia and method for obtaining synergistic T cells
A synergist and cell technology, which is used to target specific cell fusion, receptor/cell surface antigen/cell surface determinant, blood cell cancer vaccine, etc., and can solve problems such as poor treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Construction and preparation of EpCAM CAR T cells and CD33 CAR T cells
[0040] In this example, EpCAM CAR T cells and CD33 CAR T cells were prepared. Except for the difference in CAR chimeric sequence, other operations and reagents were the same for the two cells.
[0041] CAR chimeric sequence for EpCAM CAR T cells: The murine anti-human EpCAM scFv was connected to the CD8 transmembrane region, 41bb co-stimulatory domain and CD3ζ to obtain the murine anti-human EpCAM chimeric sequence (AE4 scFv-CD8-CD28- CD3ζ, whose sequence is shown in SEQ ID No: 1), was inserted into PCDH-MSCV-MCS-EF1-copGFP (purchased from Addgene).
[0042] CAR chimeric sequence of CD33 CAR T cells: The humanized anti-human CD33 scFv is connected to the CD8 transmembrane region, 41bb co-stimulatory domain and CD3ζ to obtain a humanized anti-human CD33 chimeric sequence (such as SEQ ID No: 3), inserted into PCDH-MSCV-MCS-EF1-copGFP (purchased from Addgene)
[0043] Then, the three plasm...
Embodiment 2
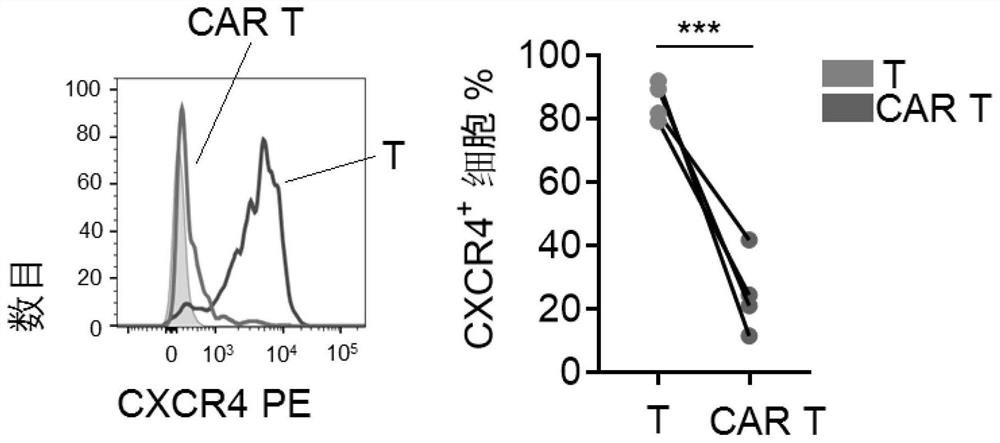
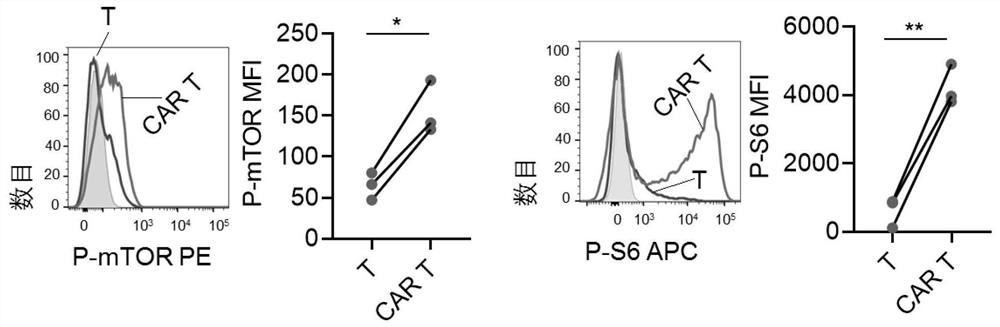
[0048] Example 2. Down-regulation of chemokines and activation of mTOR in CAR T cells cultured in vitro
[0049] Determination groups: T cells initially isolated in Example 1 (indicated by T), and EpCAM CAR T cells cultured in vitro for 5 days in Example 1 (indicated by CAR T).
[0050] Flow Cytometry
[0051] The cells to be determined were detected by BD LSRII flow cytometer and analyzed by Flowjo V10.
[0052] In the determination of this example, the anti-human antibody EpCAM was purchased from biolengend, (324208), the anti-human CXCR4 antibody was purchased from eBioscience (12-9999-42), the anti-human mTOR antibody was purchased from BD (583489), and the anti-human S6 The antibody was purchased from CST (14733S), the anti-human CD62L antibody was purchased from BD (555544), and the anti-human CD45RO antibody was purchased from BD (560607).
[0053] Wash the EpCAM T cell line harvested in vitro once in phosphate buffered saline supplemented with 2% fetal bovine serum, ...
Embodiment 3
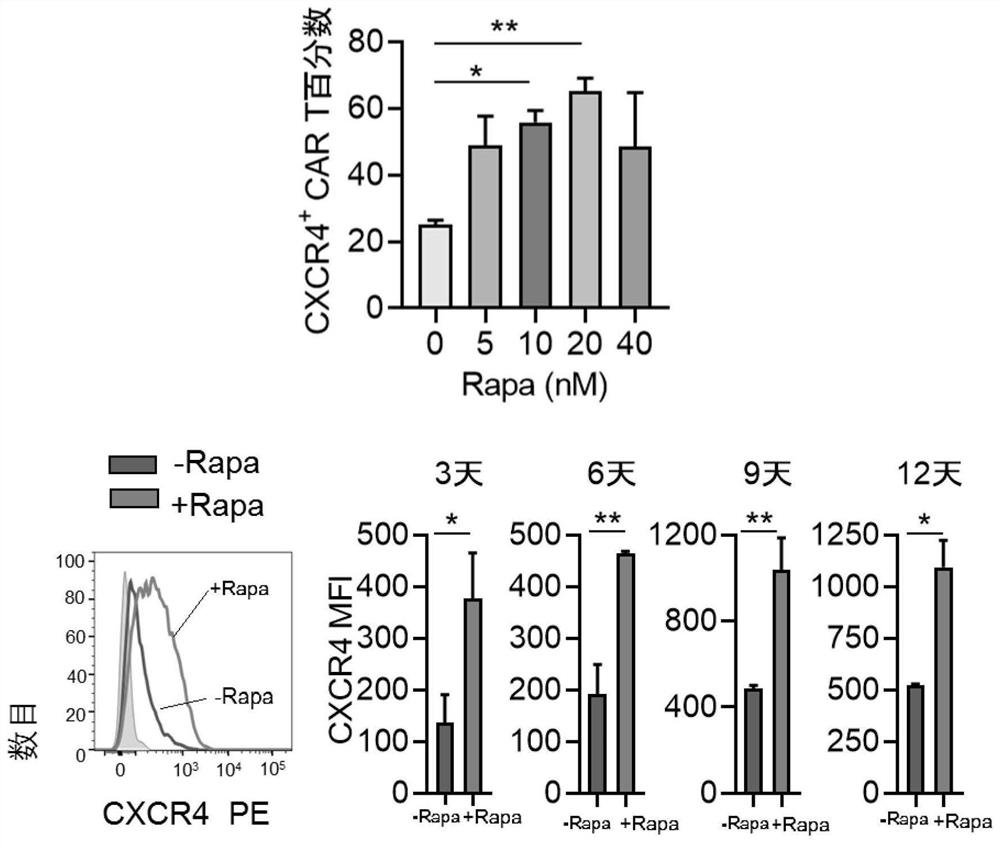
[0057] Example 3. Effect of rapamycin treatment on chemokines of EpCAM CAR T cells
[0058] Determination groups: EpCAM CAR T cells cultured in vitro and treated with rapamycin at 24 hours (indicated by +Rapa), EpCAM CAR T cells cultured in vitro but not treated with rapamycin (indicated by -RaPa). Sample collection time points: Days 3, 6, 9, and 12.
[0059] Concentration screening: T cells were isolated and transfected with lentivirus as in Example 1, and CAR T cells were treated with 0nM, 5nM, 10nM, 20nM, and 40nM rapamycin respectively, and the expression of CXCR4 was detected by flow cytometry after 6 days of culture. The results are shown in image 3 .
[0060] The results showed that 5nM, 10nM, 20nM, and 40nM can all be used to treat CAR T cells, and 20nM is the optimal concentration, which can most effectively up-regulate the expression of CXCR4.
[0061] T cells were isolated as in Example 1 and subjected to lentiviral transfection and rapamycin (20nM) treatment to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com