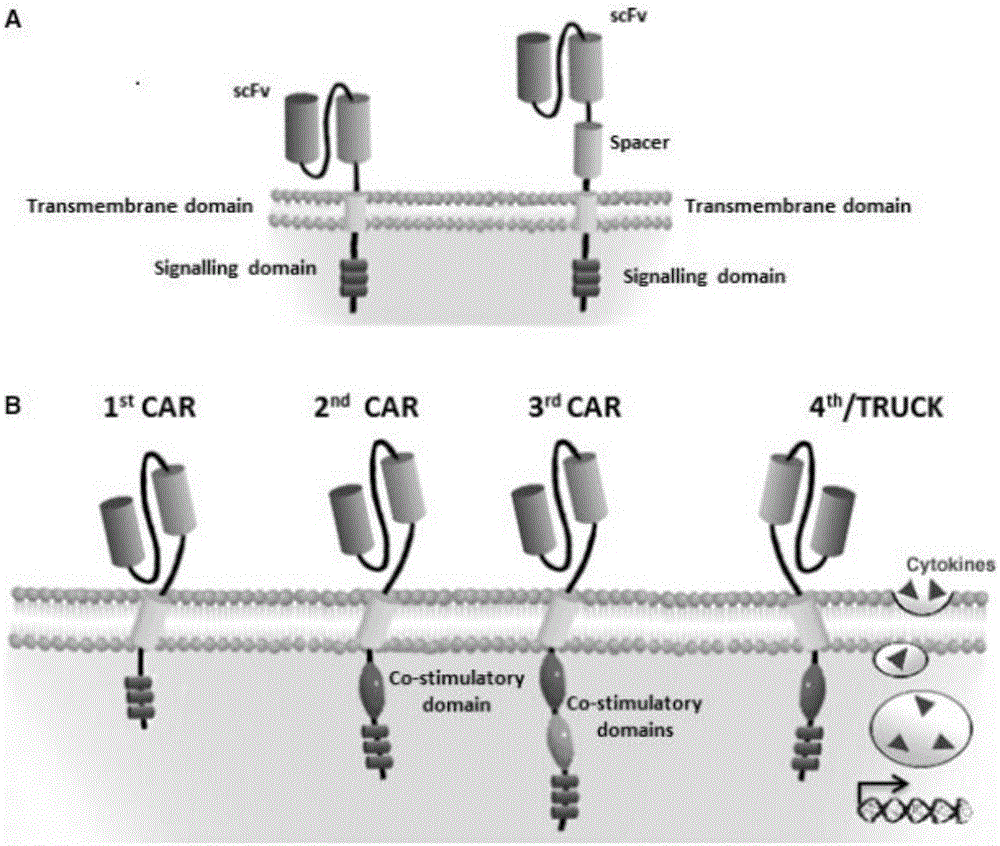
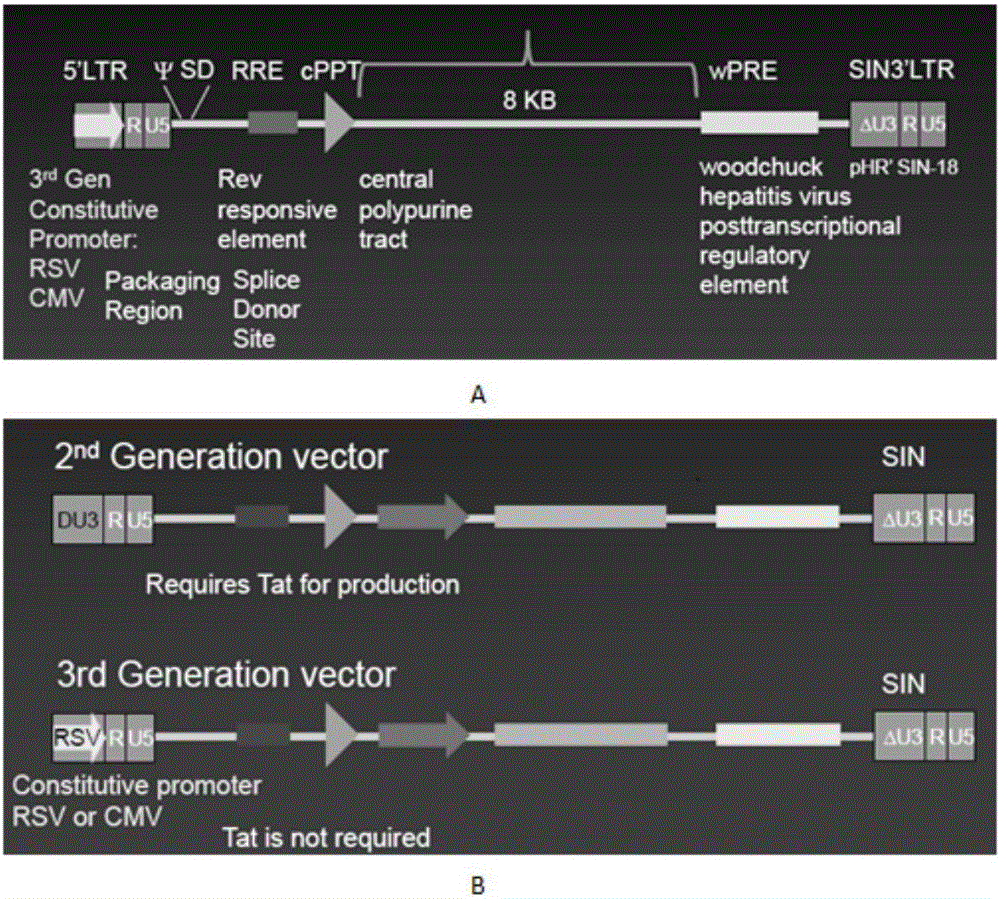
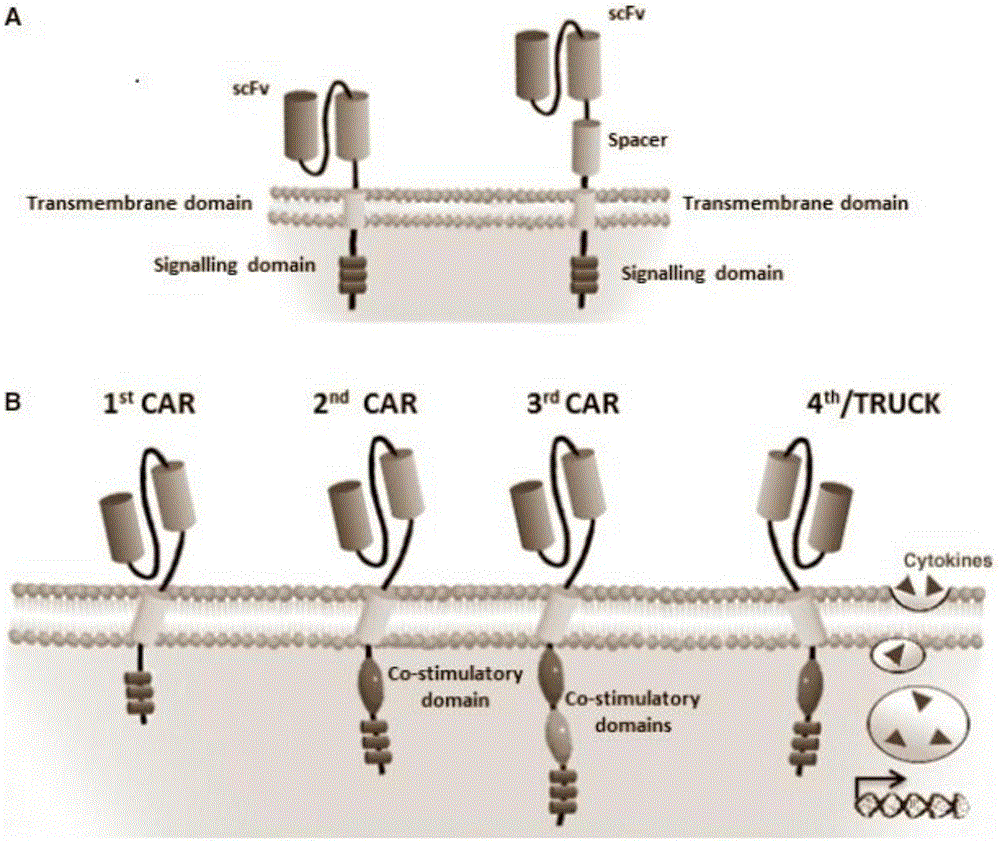
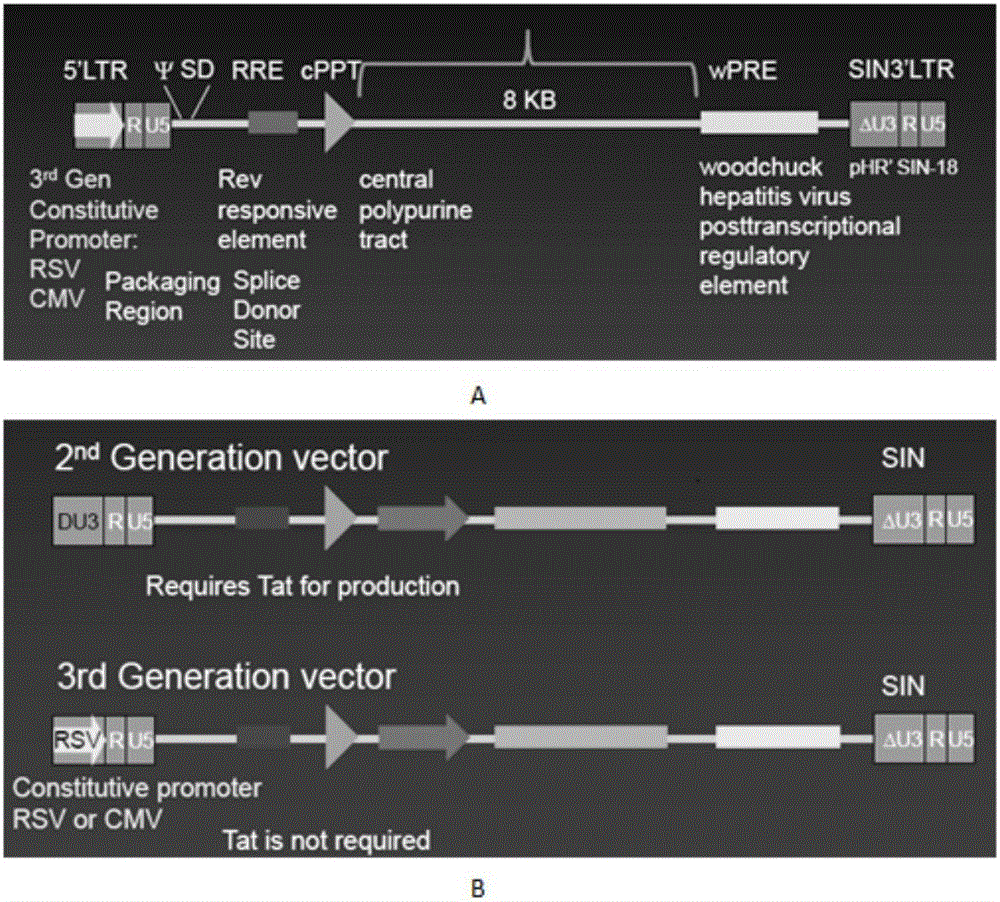
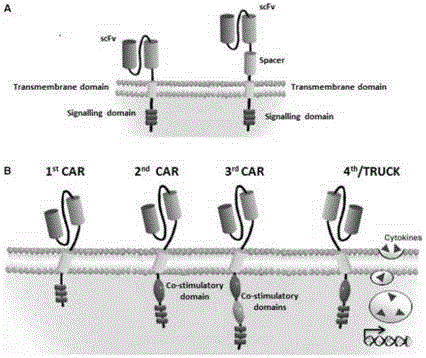
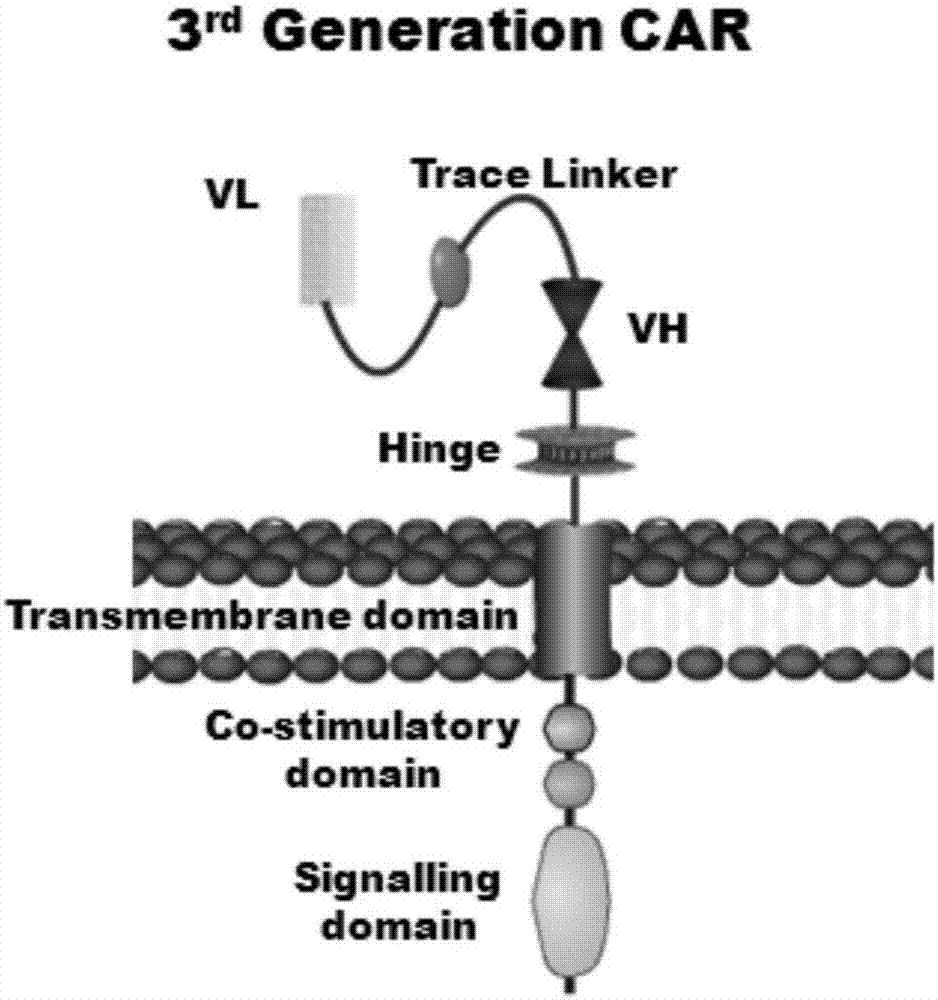
Patents
Literature
38results about How to "Eliminates the possibility of self-replication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
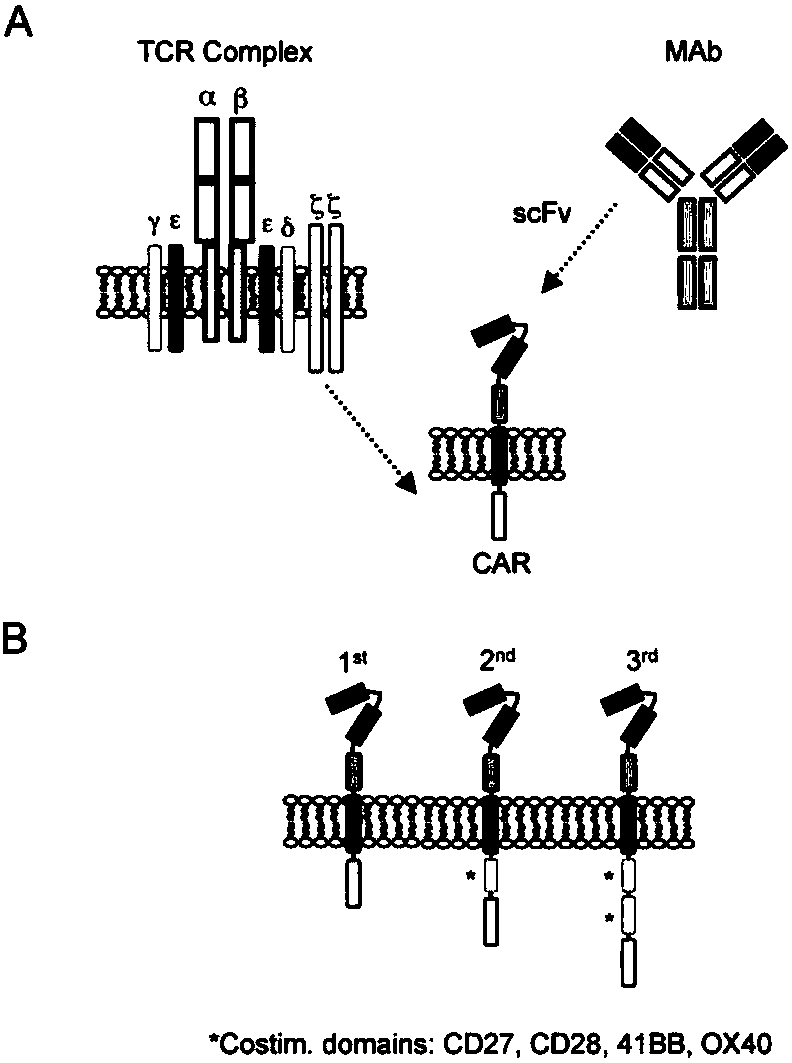
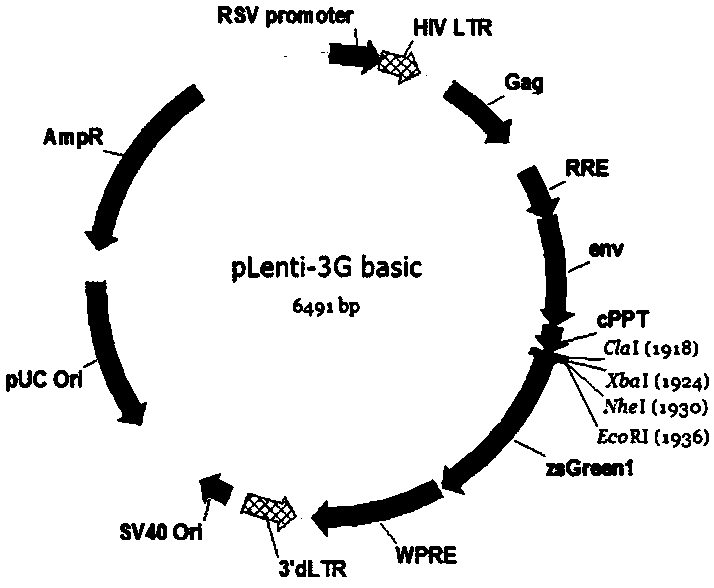
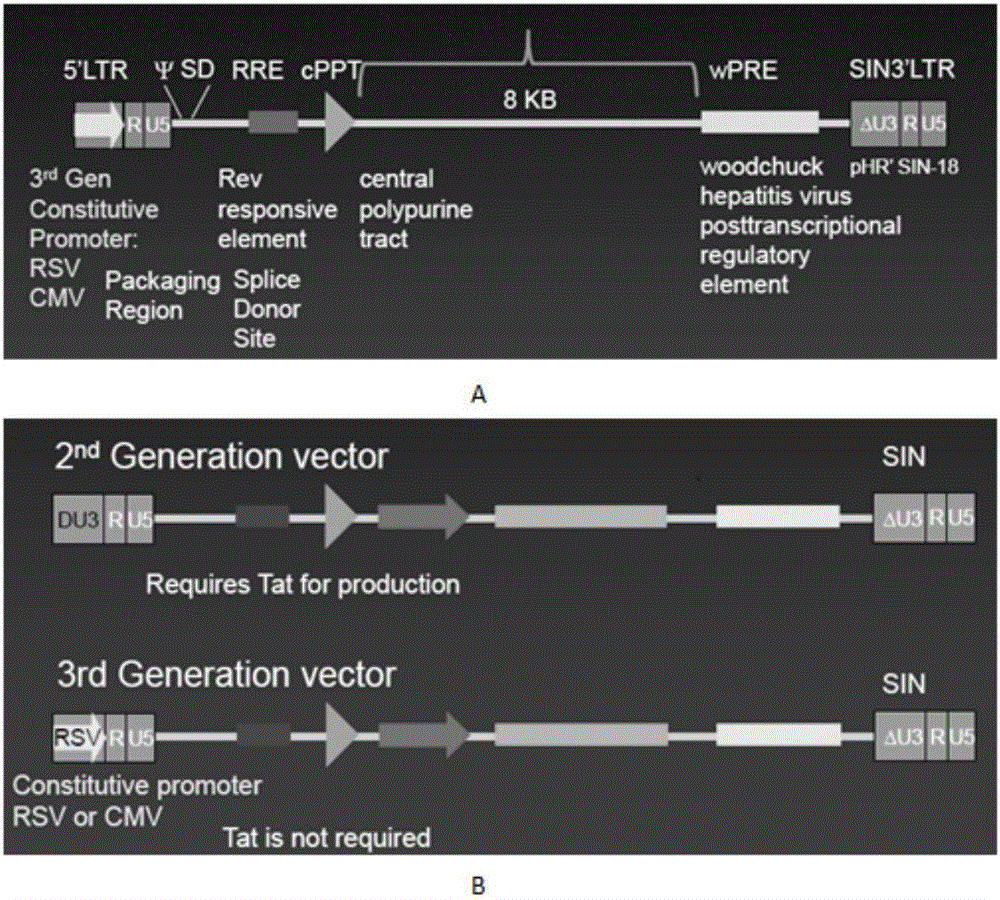
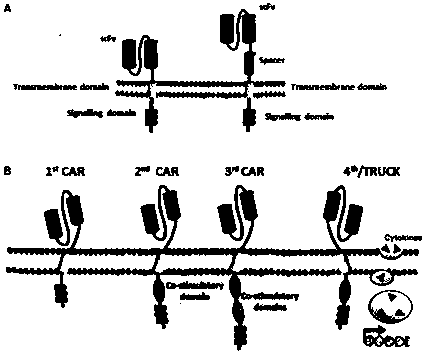
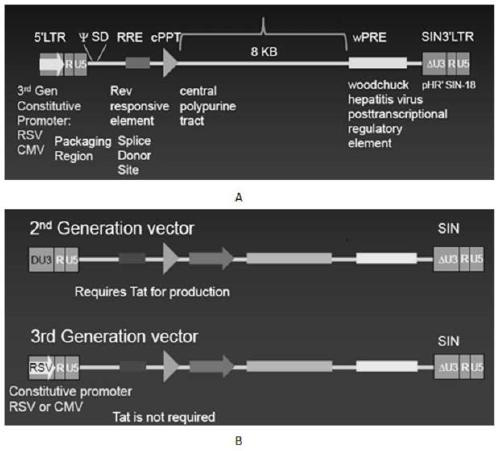
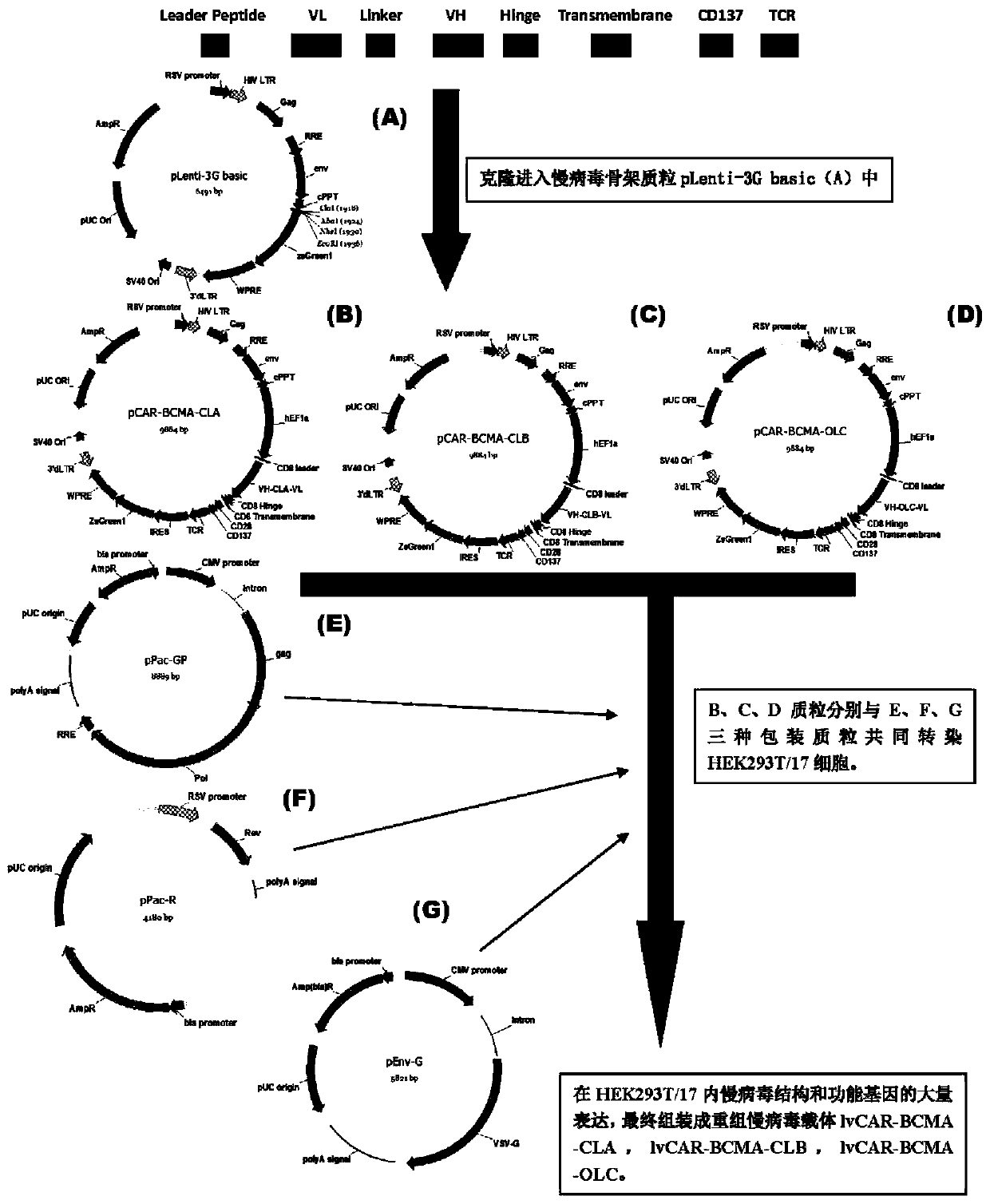
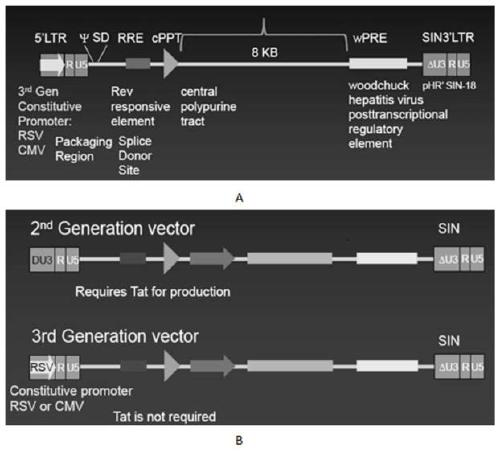
CAR-T transgene vector based on replication defective recombinant lentivirus and construction method and application of CAR-T transgene vector
ActiveCN105602992ASignificant effectPromote secretionGenetic material ingredientsFermentationEucaryotic cellAmpicillin
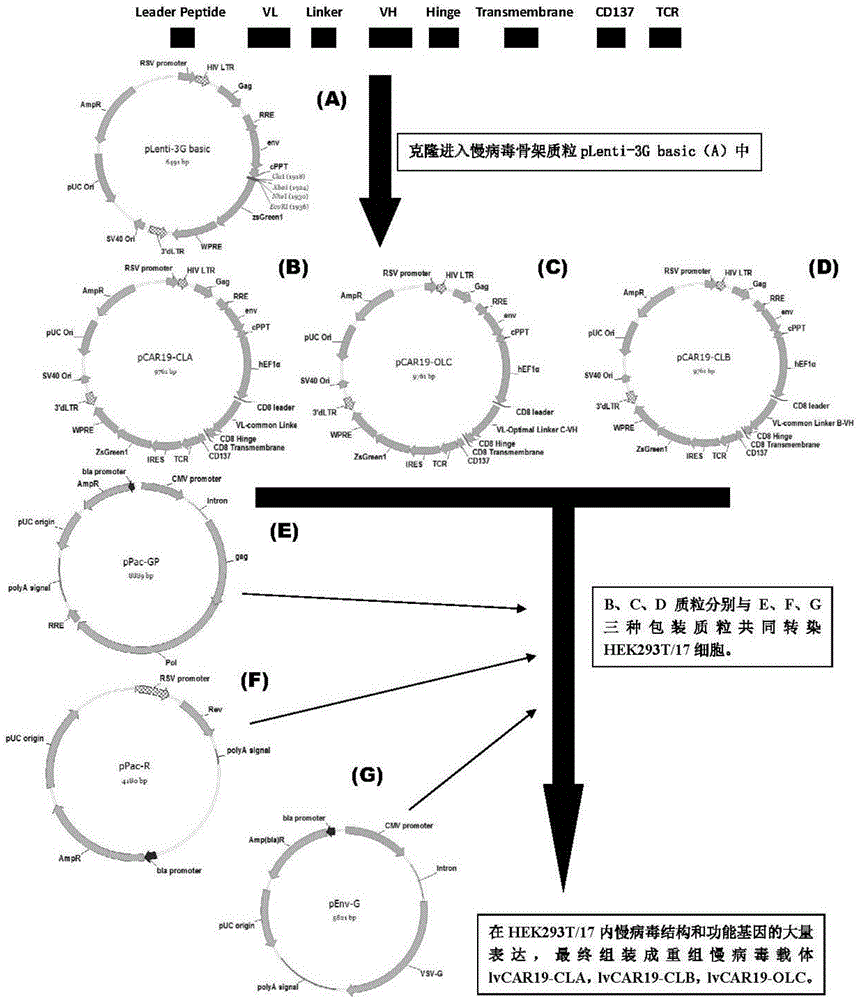
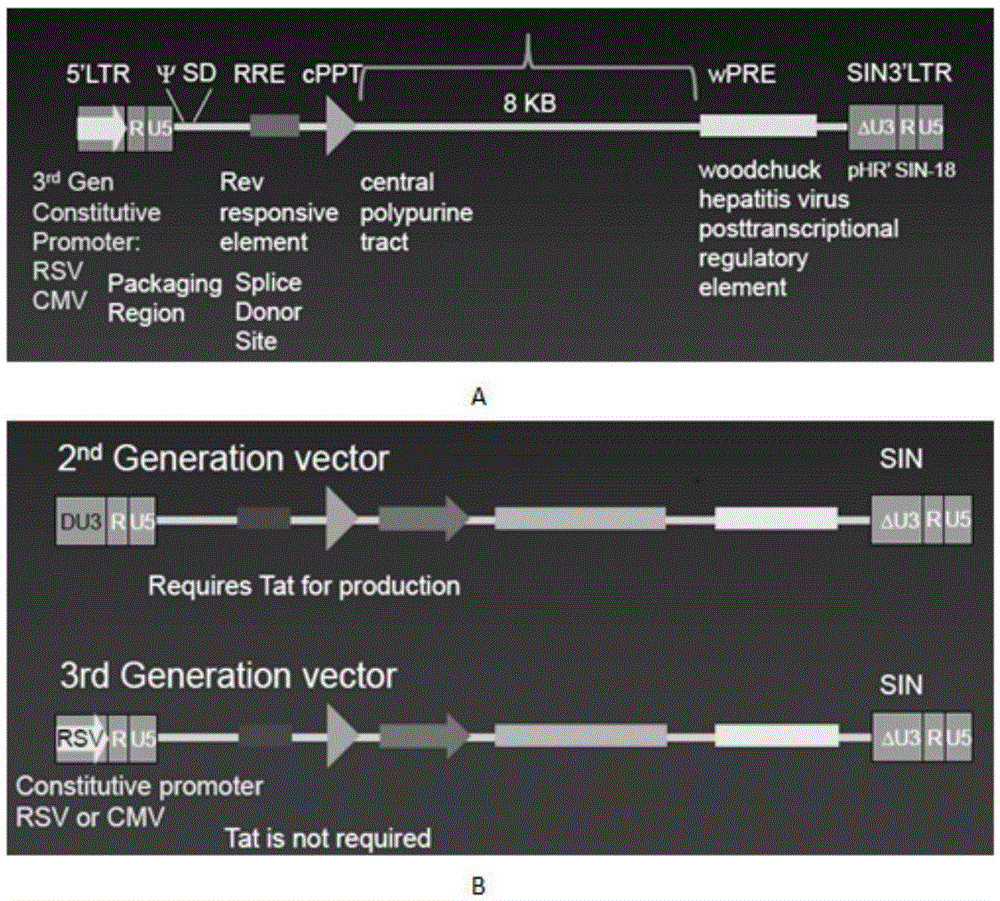
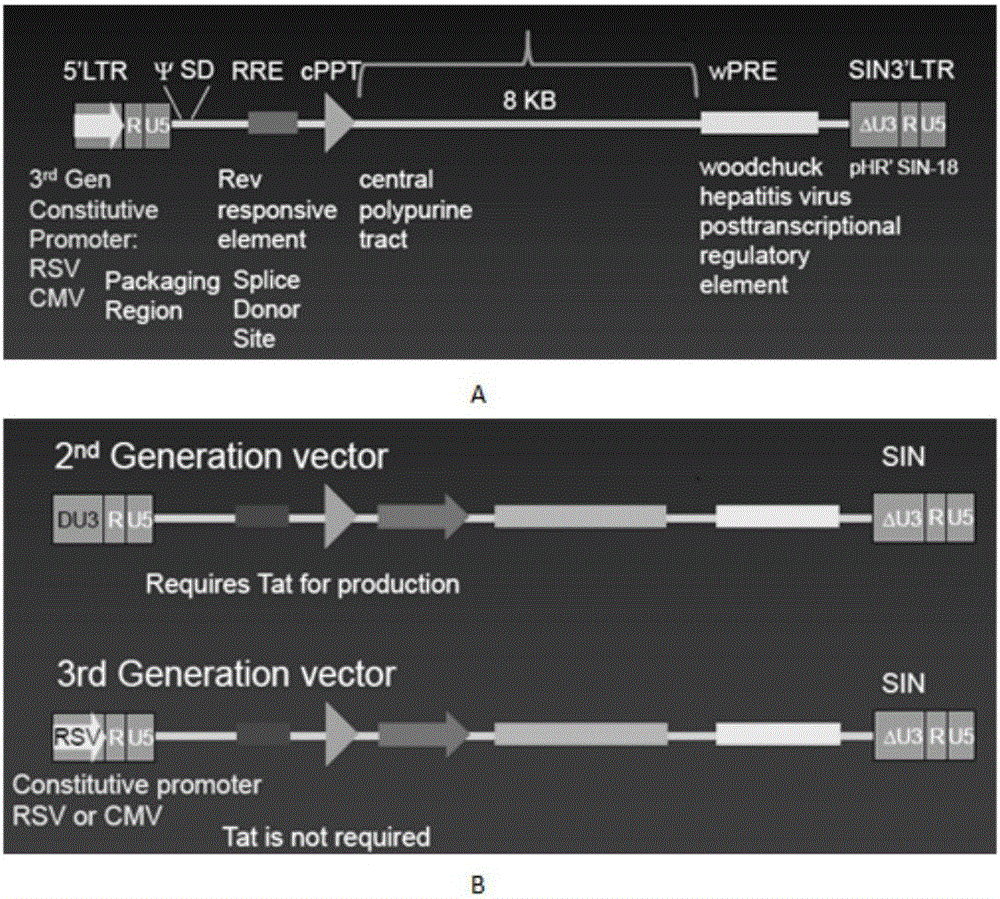
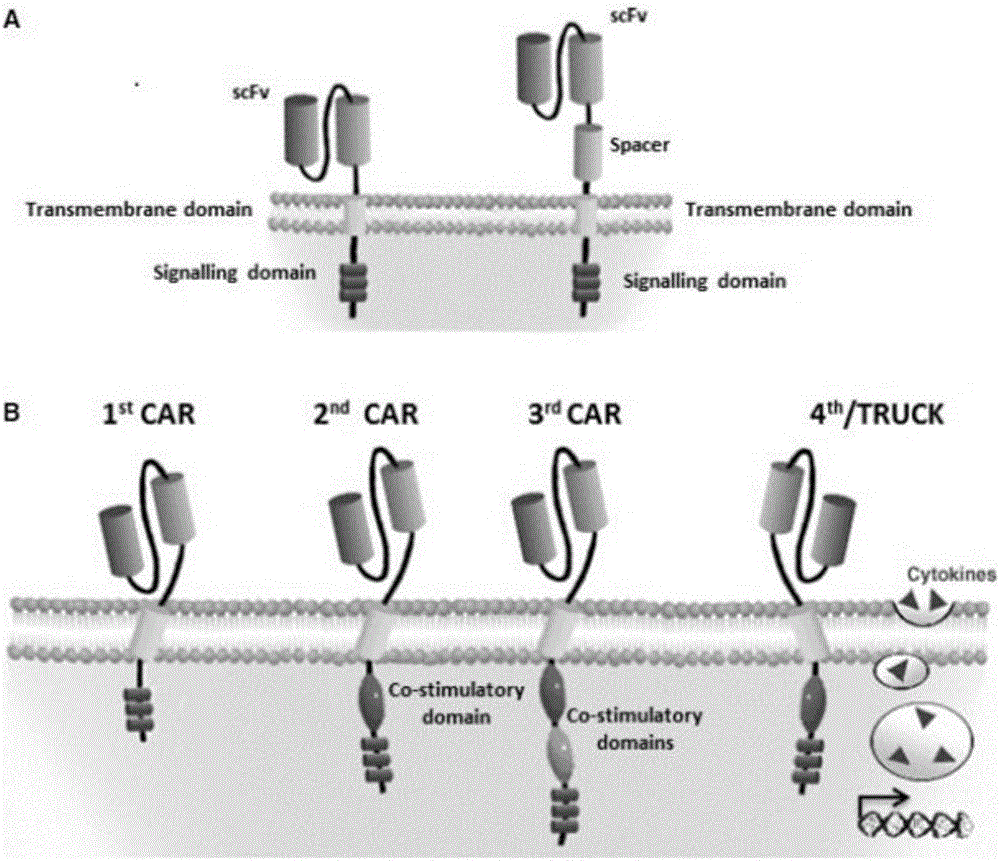
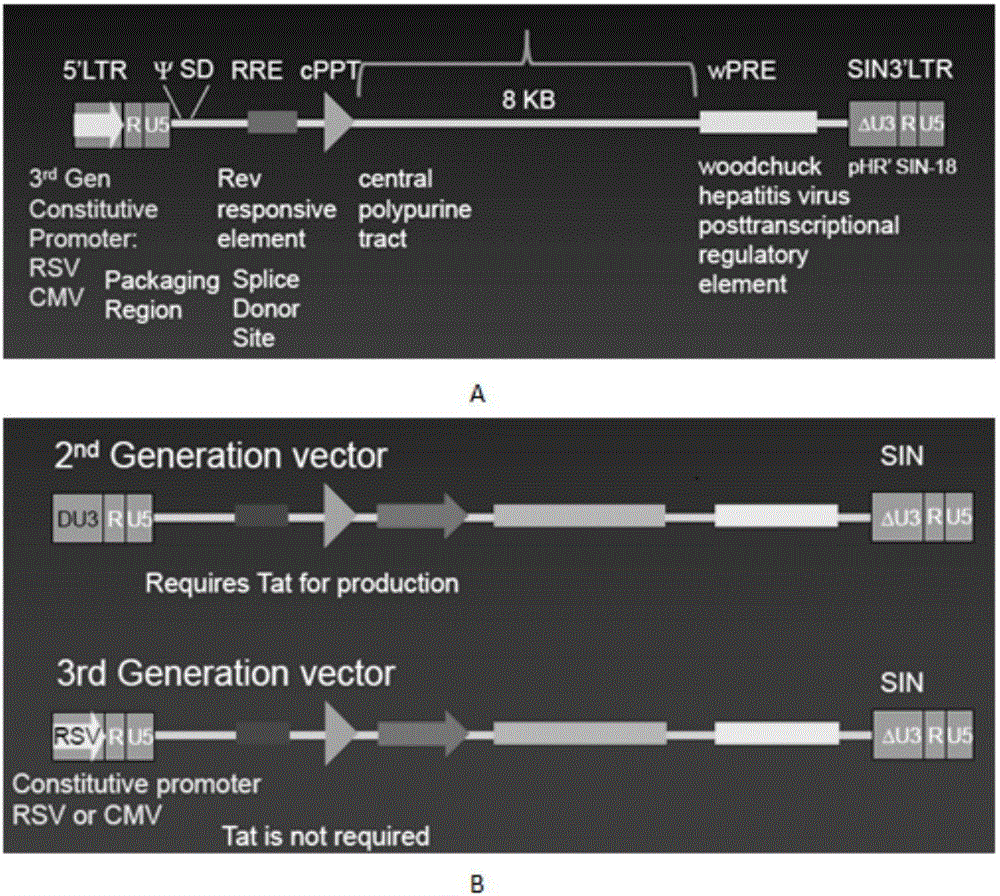
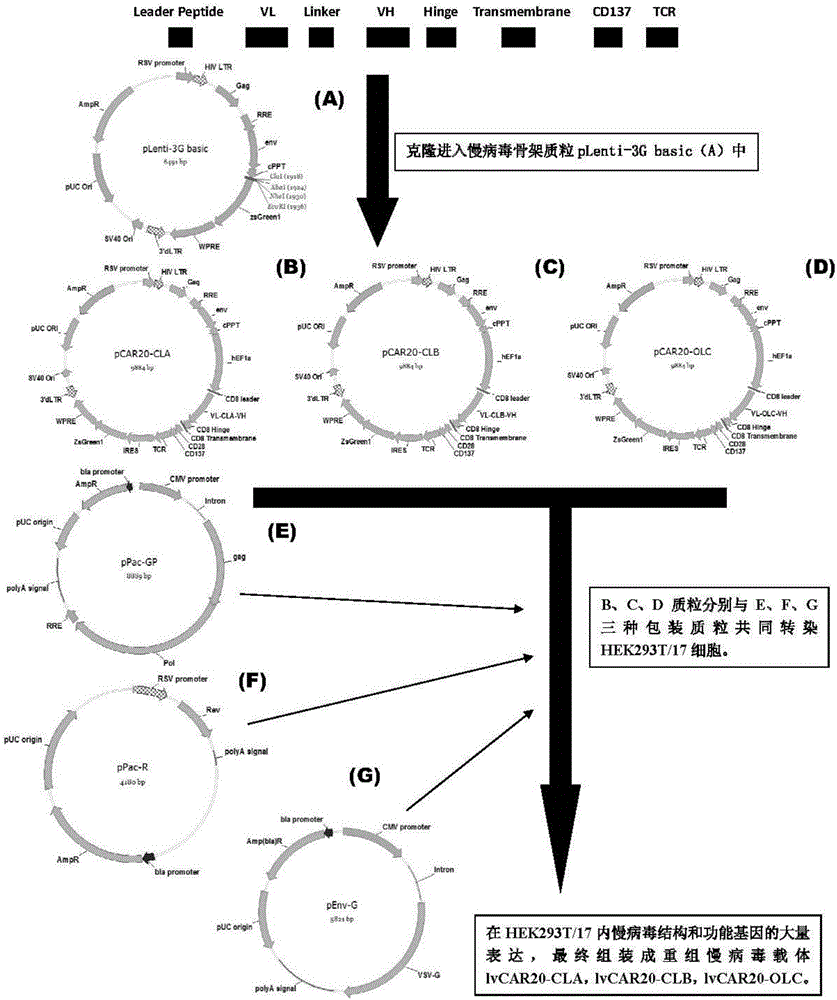
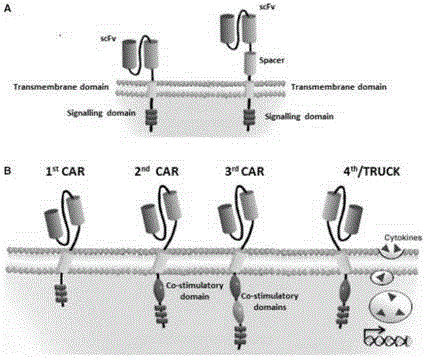
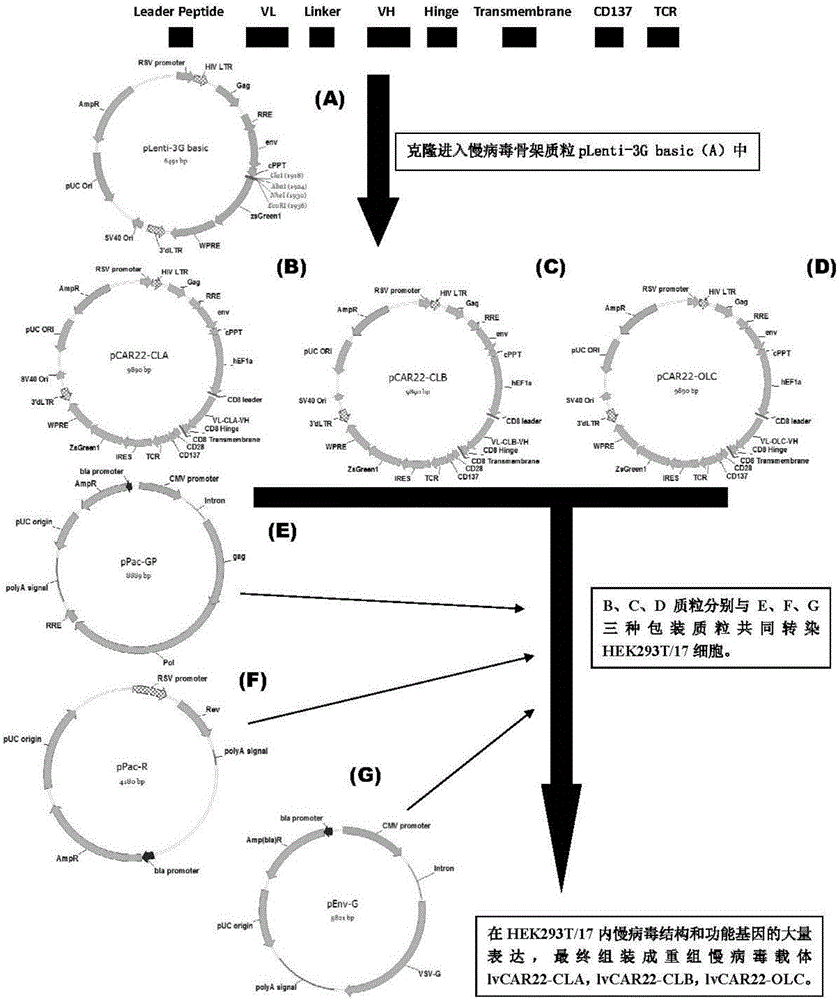
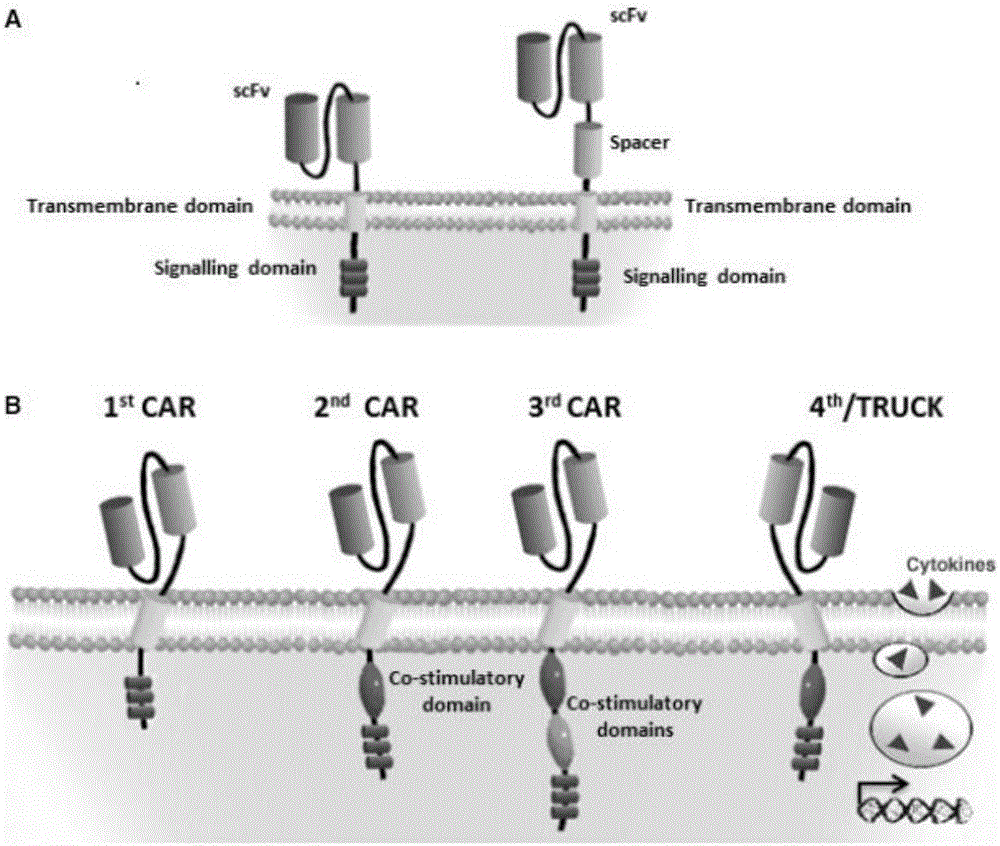
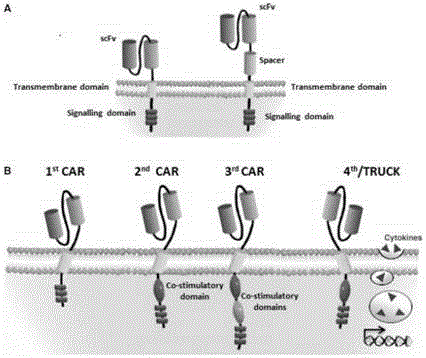
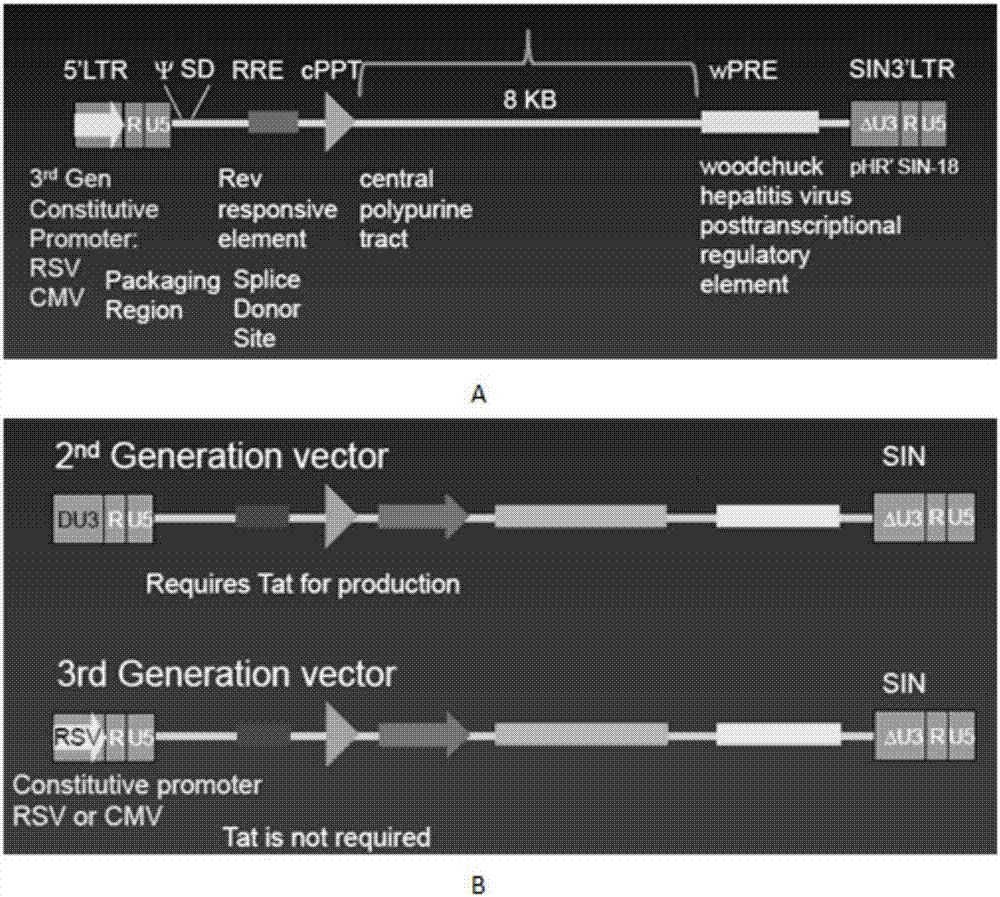
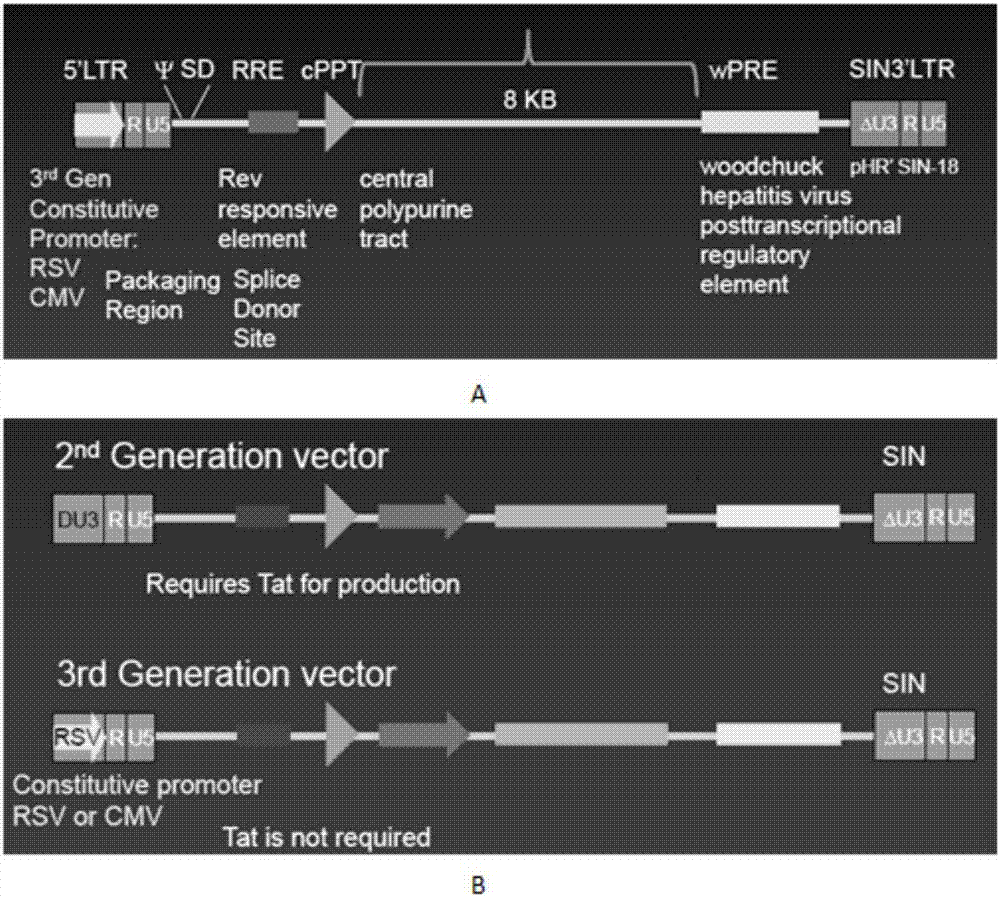
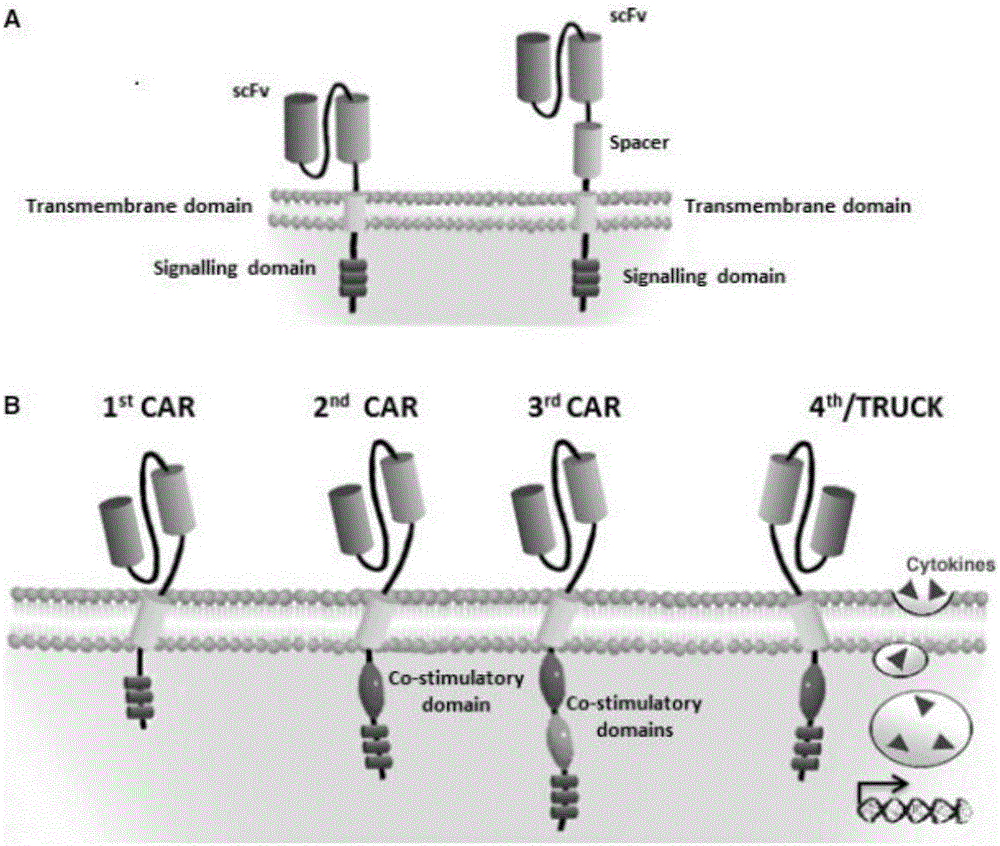
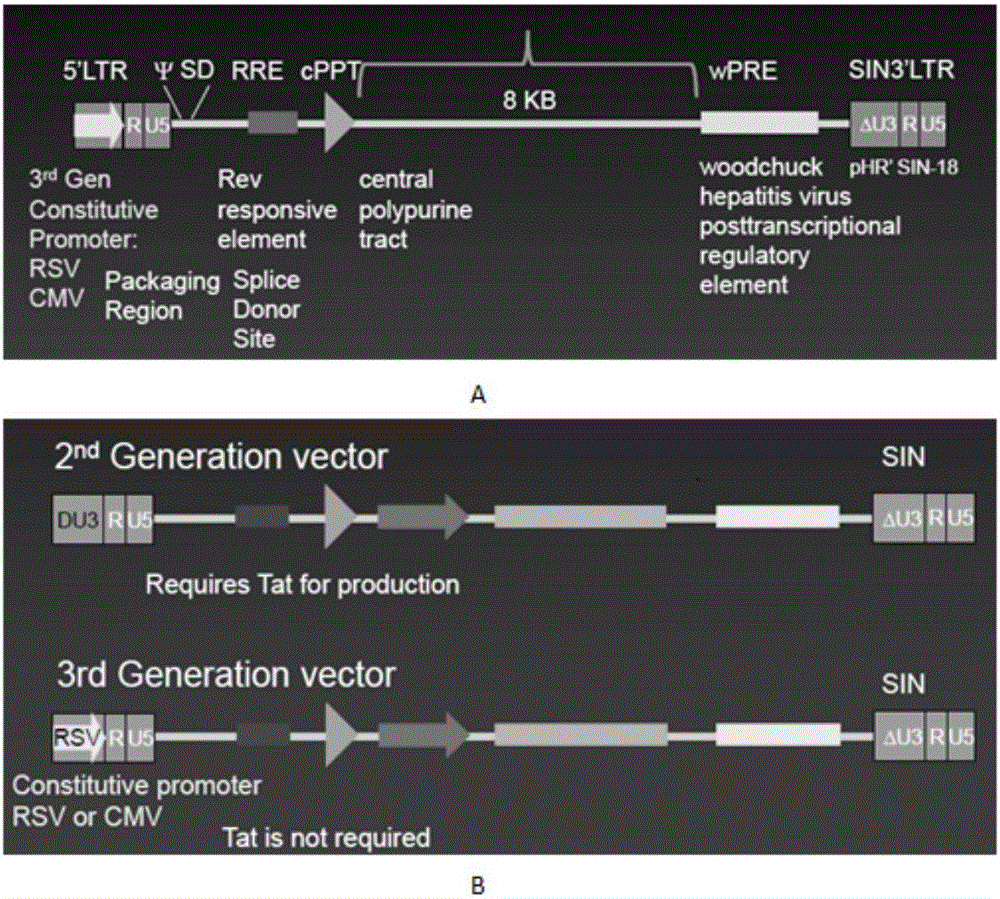
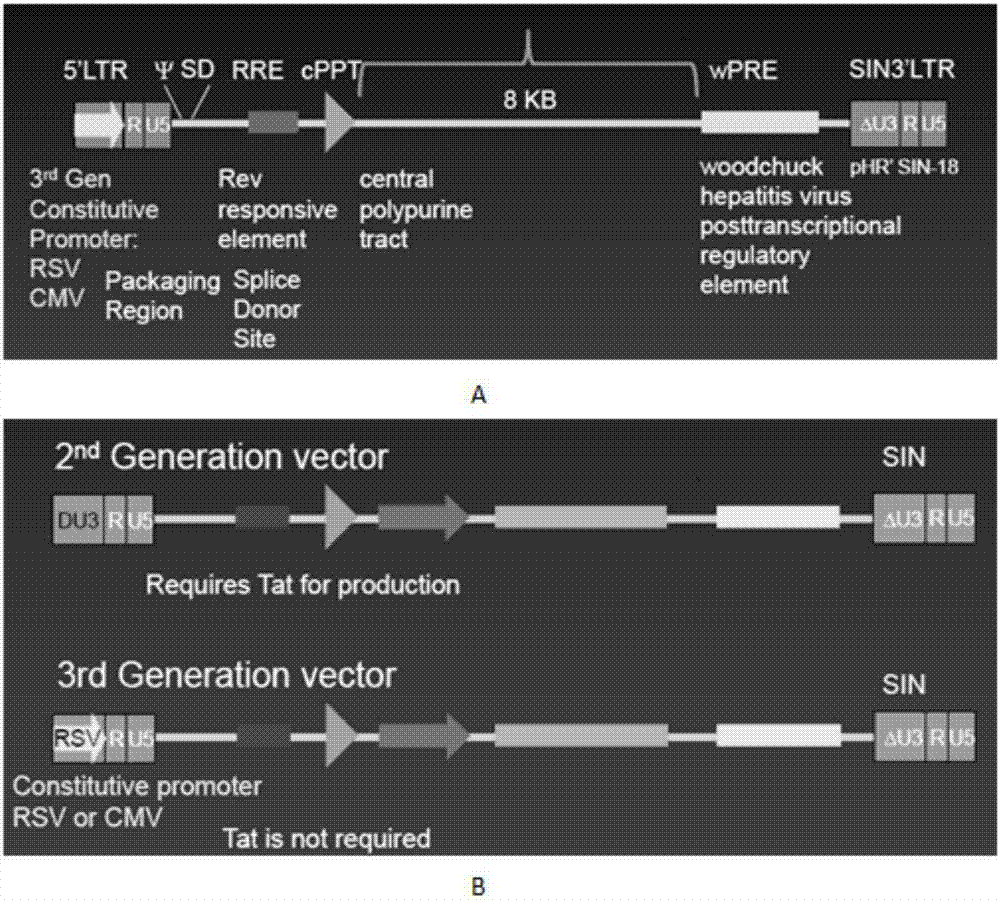
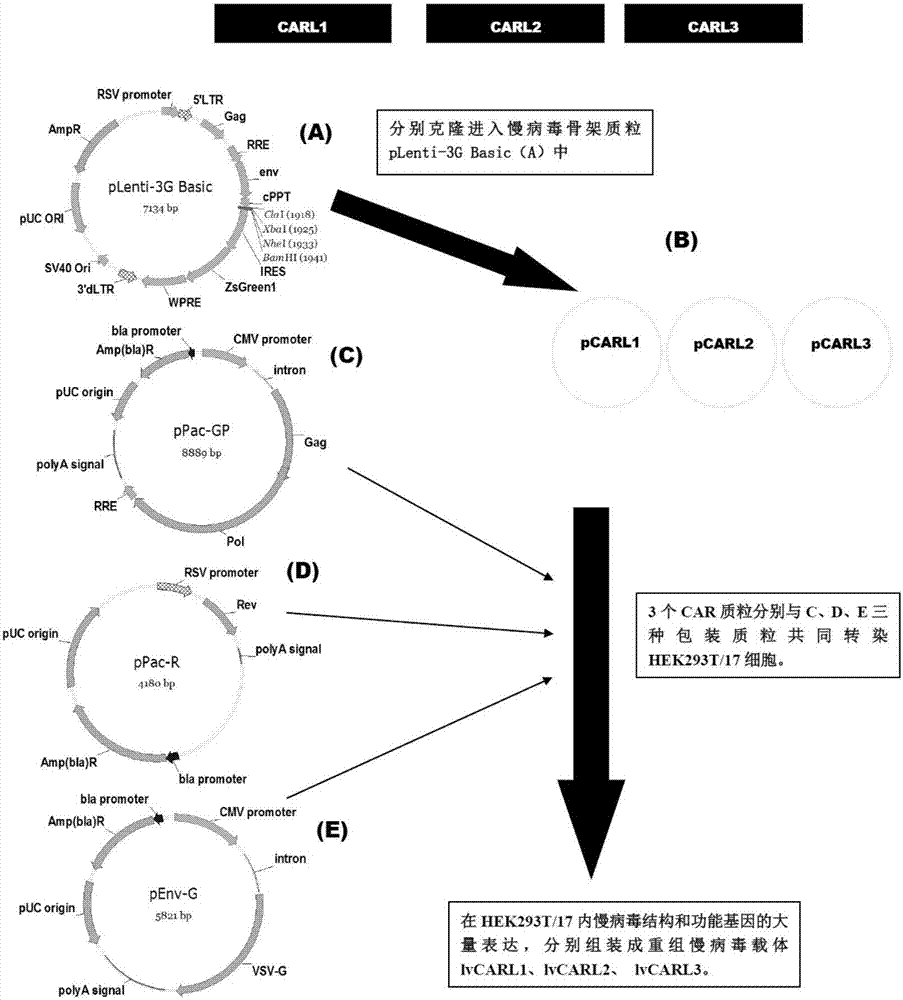
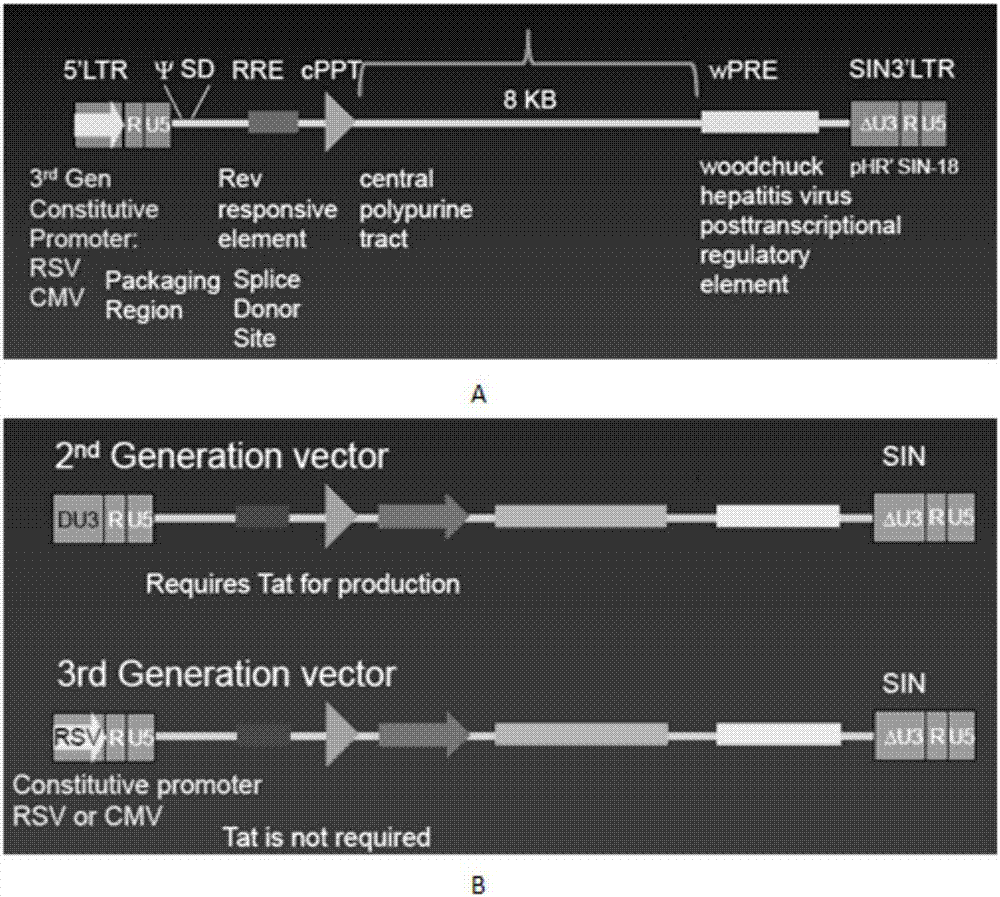
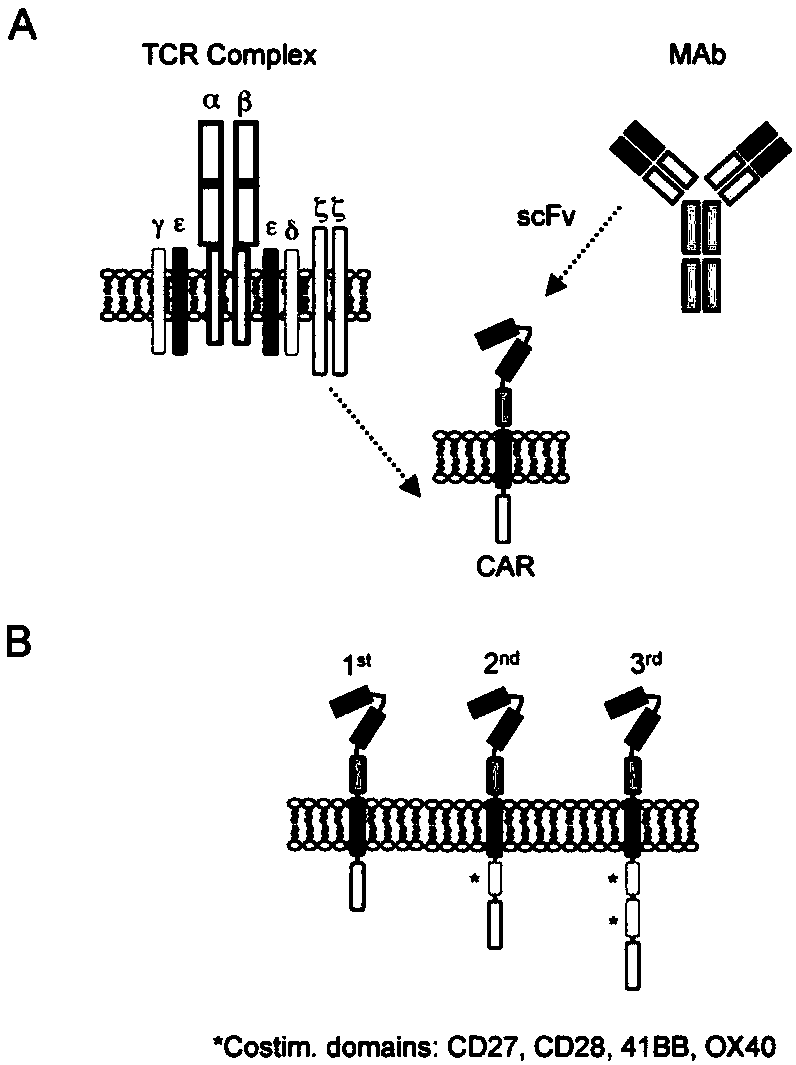
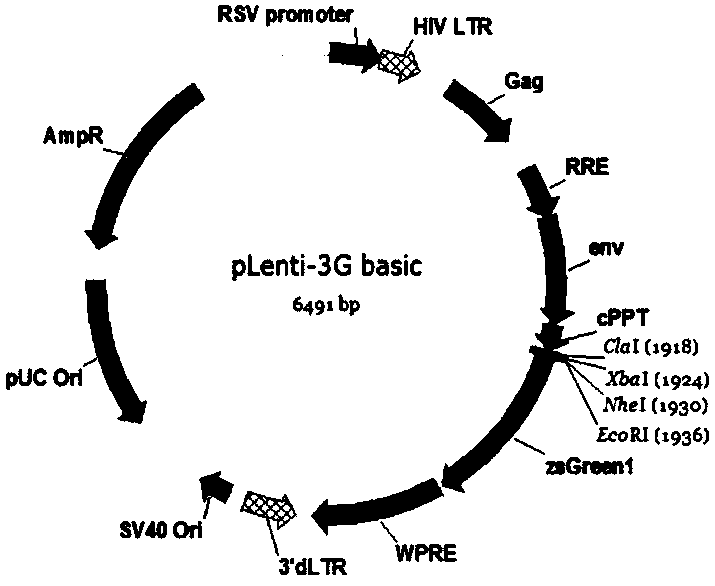
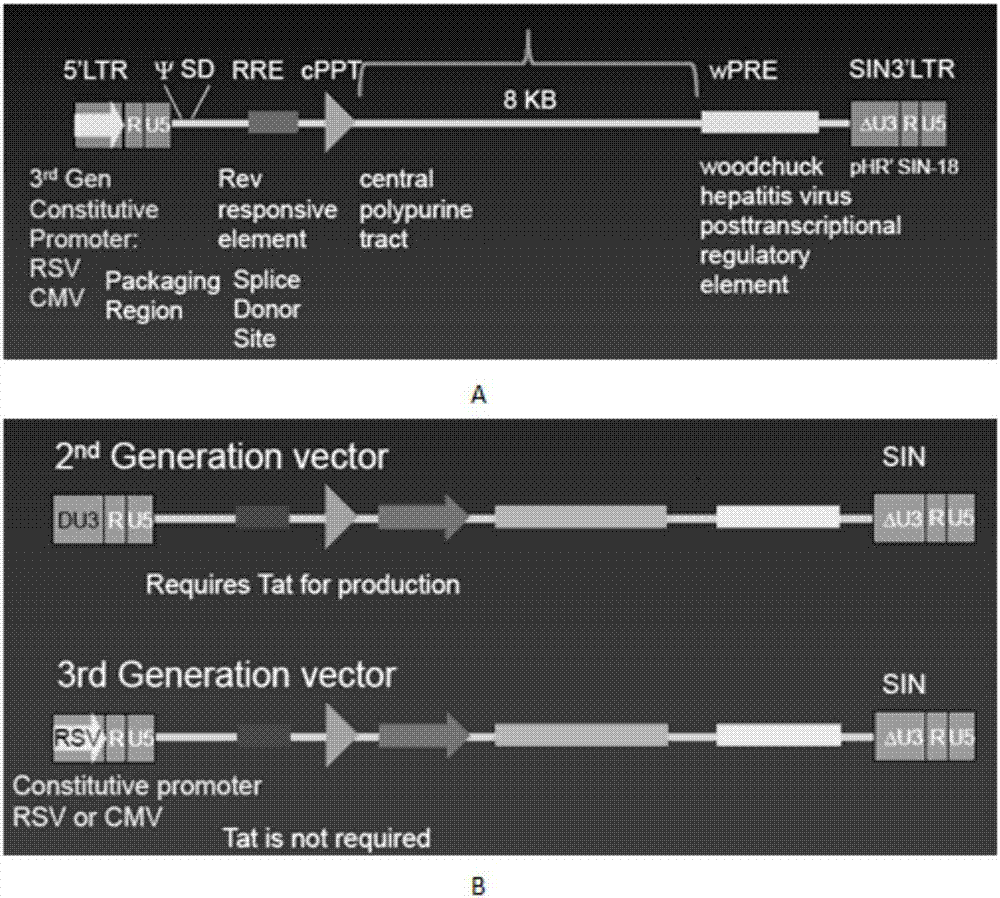
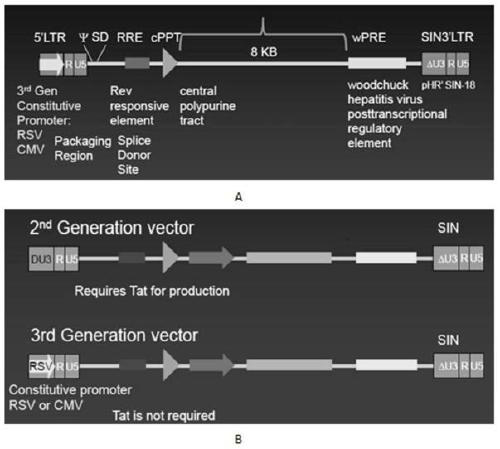
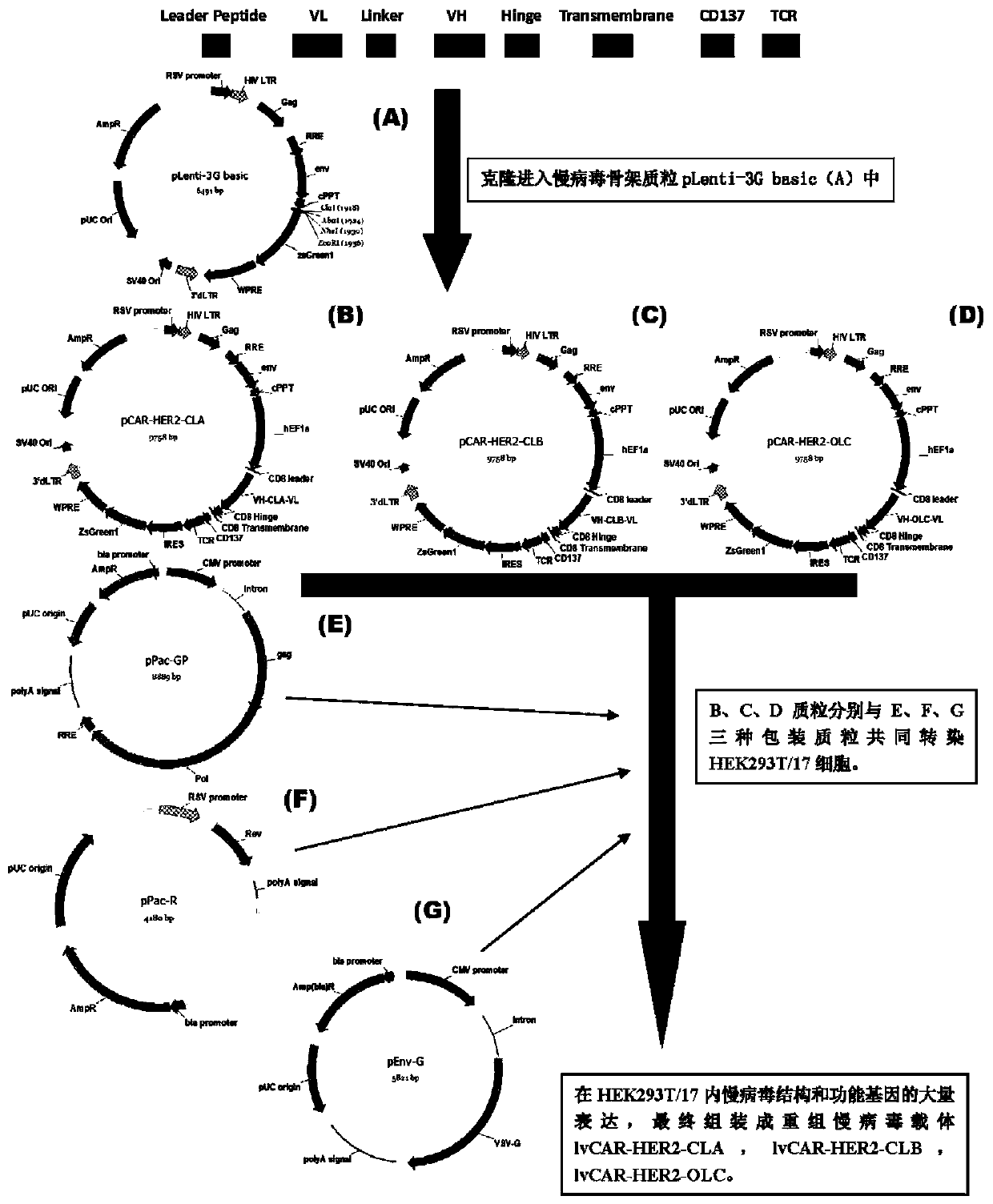
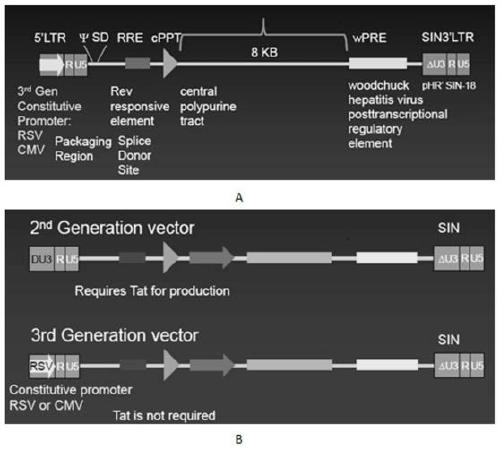
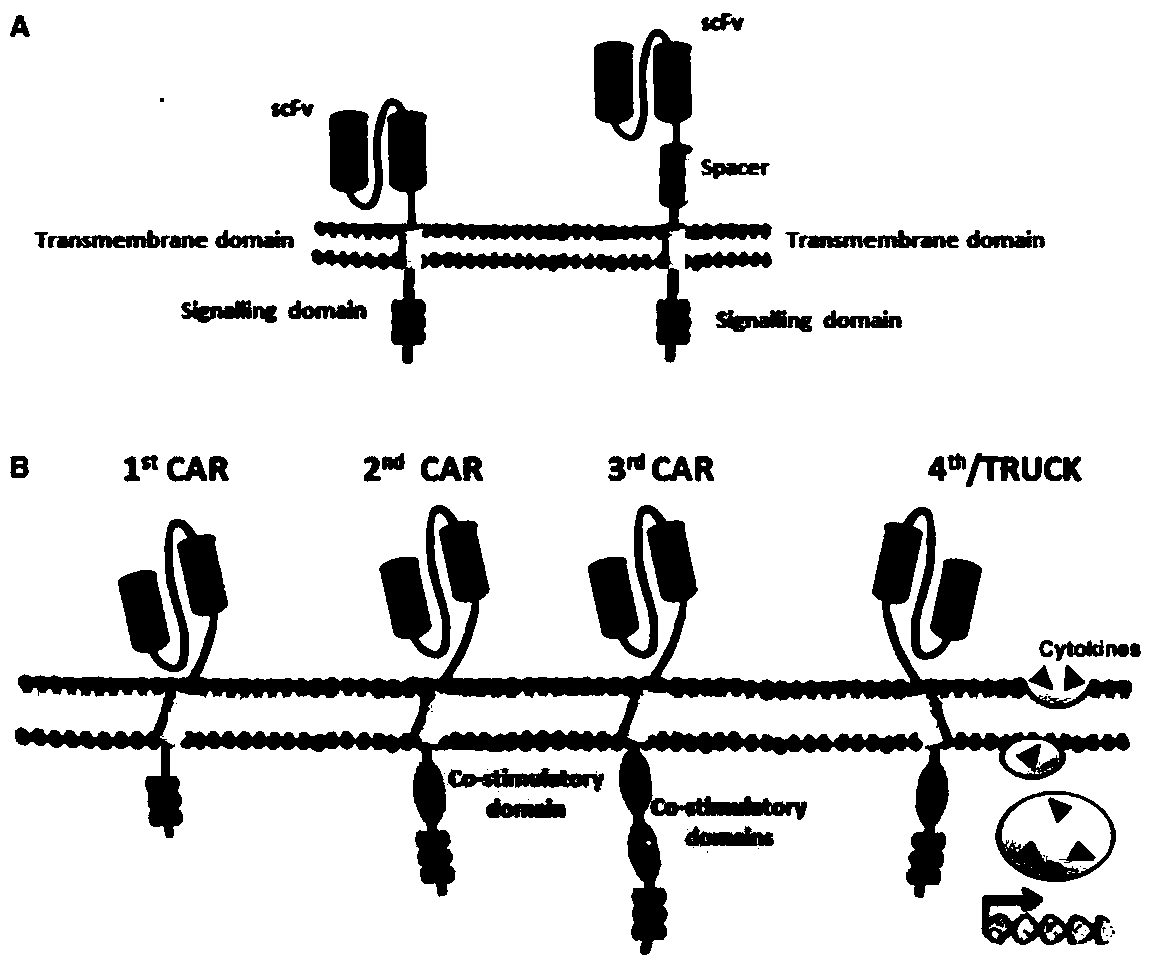
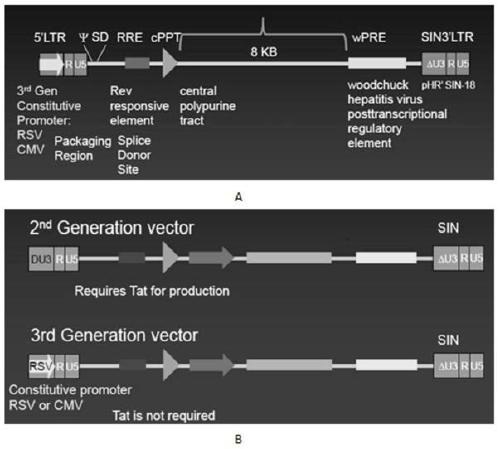
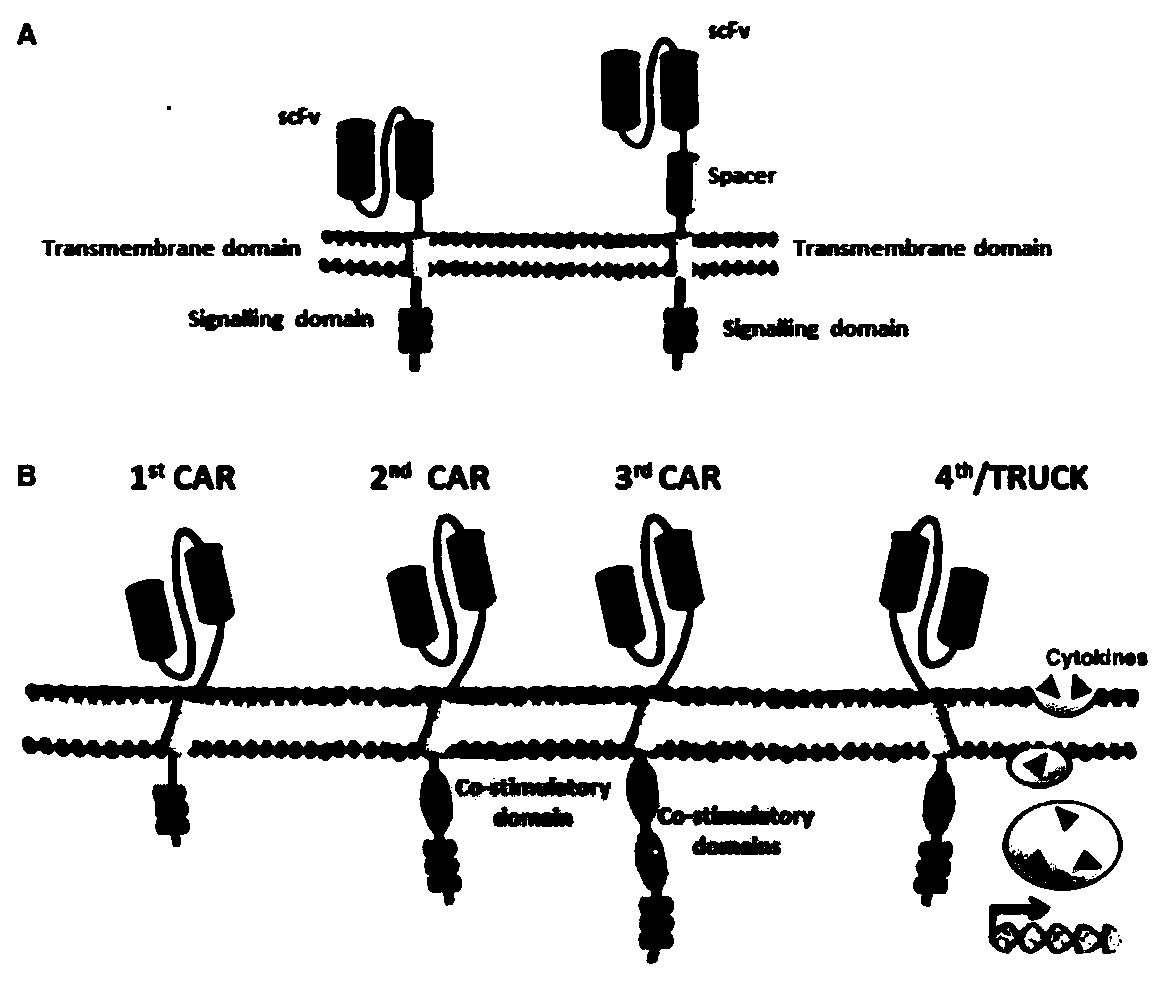
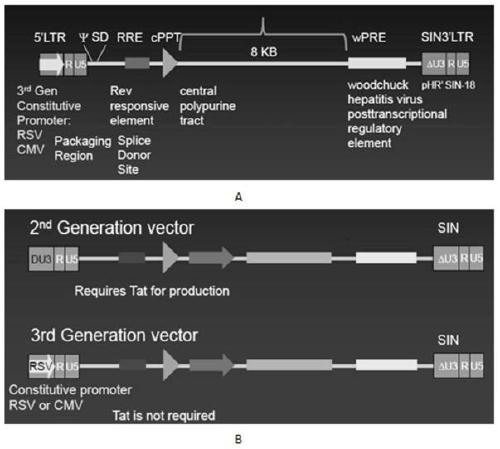
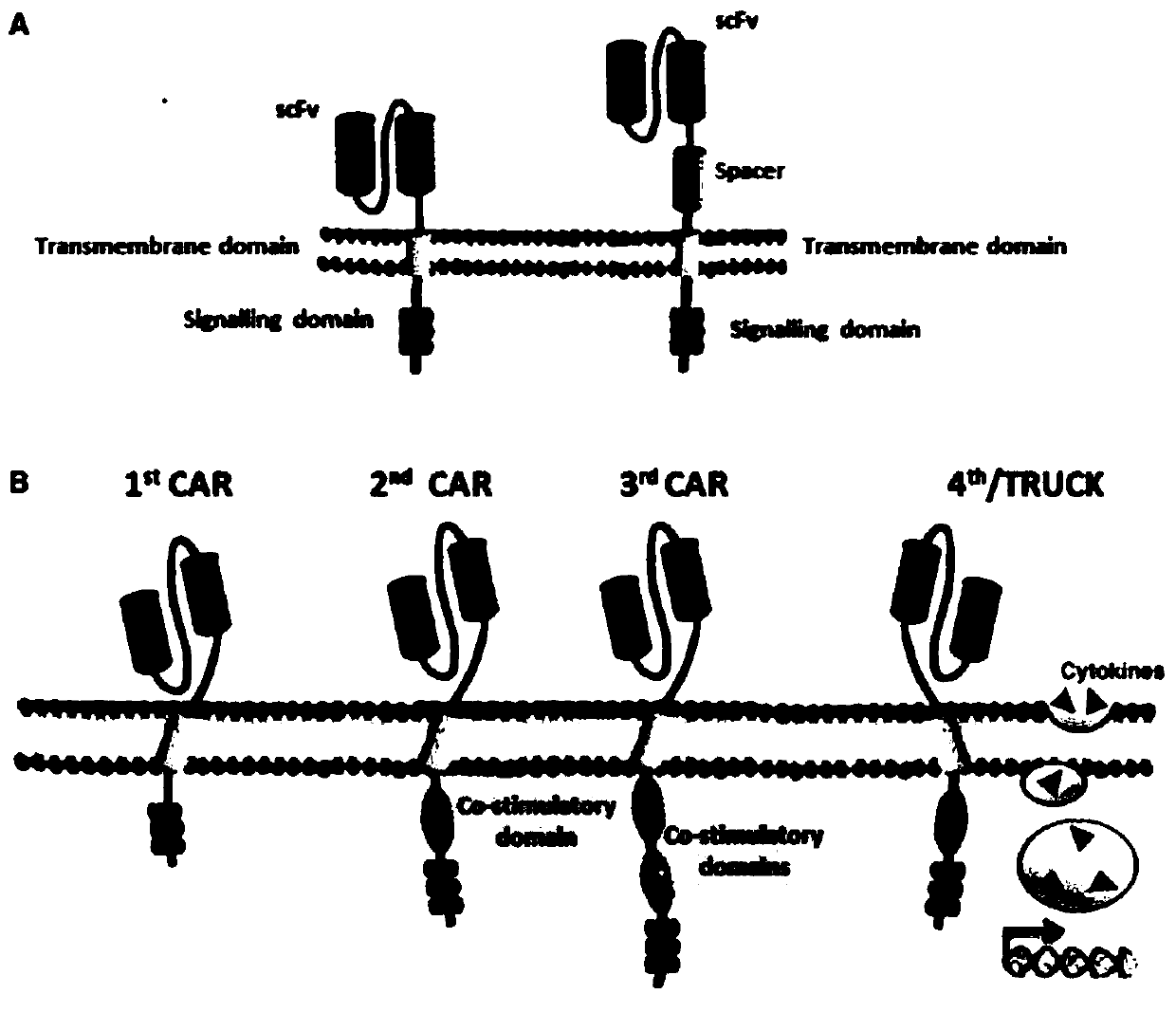
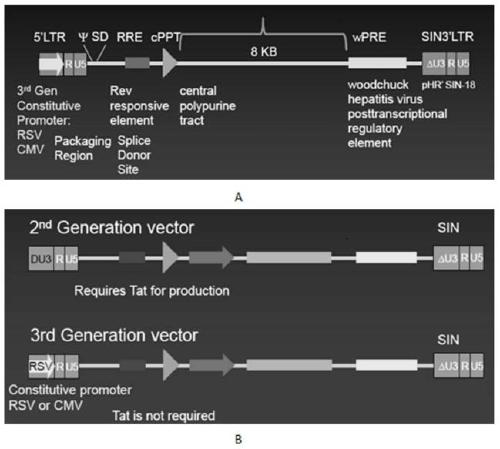
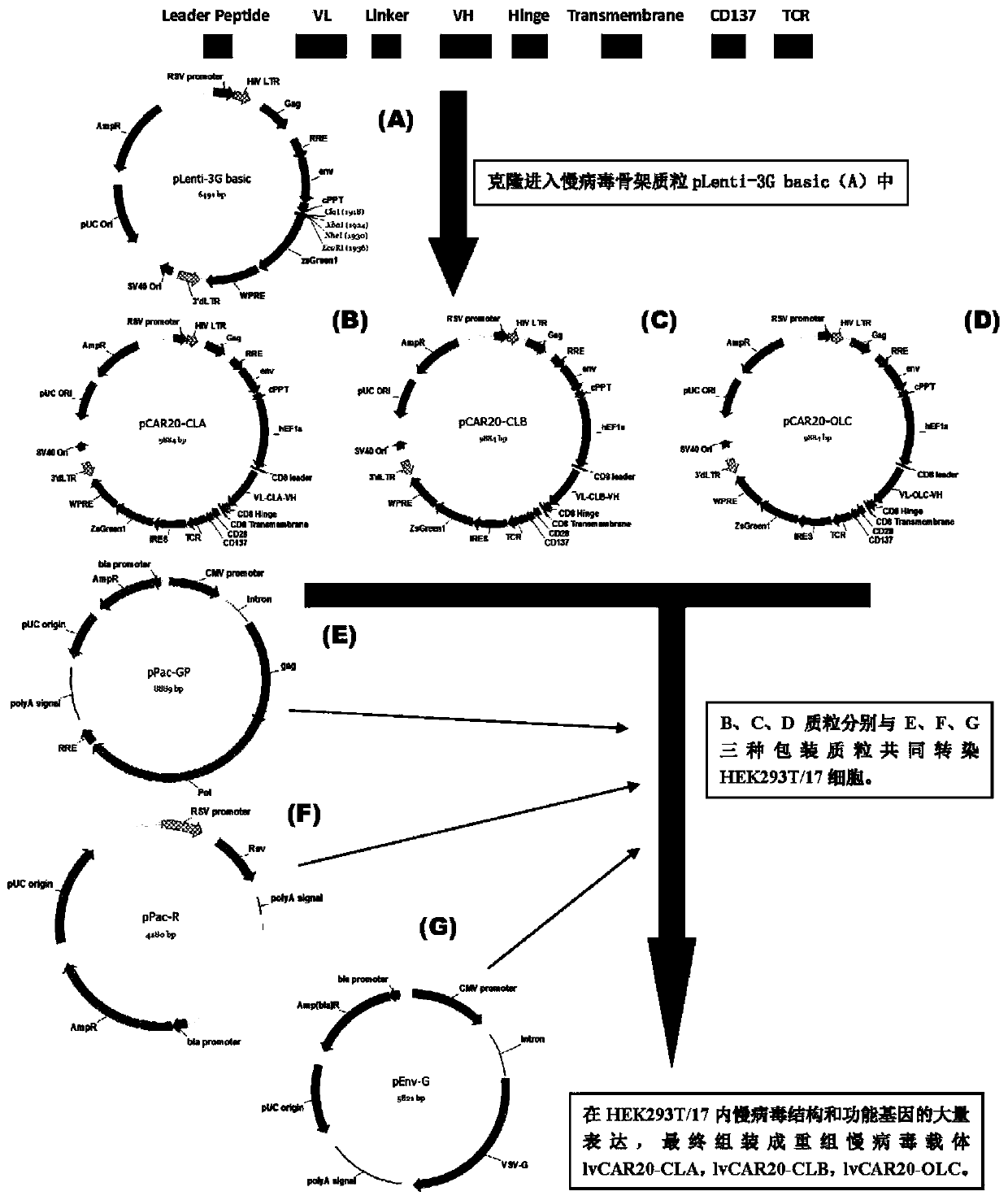
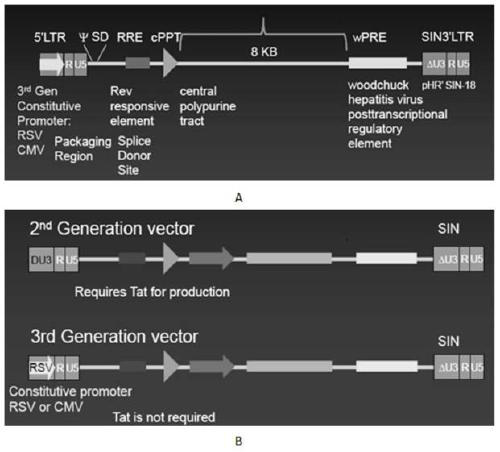
The invention discloses a CAR-T transgene vector based on replication defective recombinant lentivirus. The CAR-T transgene vector comprises an original nuclear replicon pUCOri sequence, a resistance gene AmpR sequence containing ampicillin, a virus replicon SV40 Ori sequence, a lentivirus packaging cis element, ZsGreen1 green fluorescent protein, an IRES ribosome binding sequence, a human EF1 alpha promoter , a chimeric antigen receptor of second-generation CAR or third-generation CAR and a regulating element, wherein the original nuclear replicon pUCOri sequence is used for plasmid replication; the resistance gene AmpR sequence is used for massively proliferating target strains; the virus replicon SV40 Ori sequence is used for enhancing replication in eukaryocyte; the lentivirus packaging cis element is used for lentivirus packaging; the ZsGreen1 green fluorescent protein is used for expressing green fluorescent for eukaryocyte; the IRES ribosome binding sequence is used for jointly transcribing and expressing protein; the human EF1 alpha promoter is used for conducting eukaryotic transcription on antigen receptor genes; the chimeric antigen receptor is used for forming the second-generation CAR or the third-generation CAR integrating recognition, transfer and start; the regulating element is used for enhancing expression efficiency of transgenes and used after eWPRE-enhanced type woodchuck hepatitis b virus is transcribed. Besides, the invention further discloses a construction method and application of the vector. By means of the CAR-T transgene vector and the construction method and application of the vector, secretion of cell factors and an in vitro killing effect of CAR-T cells can be remarkably improved, and the clinical treatment effect is remarkable.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Anti-BCMA chimeric antigen receptor, encoding gene, recombinant expression vector and establishing method and application of anti-BCMA chimeric antigen receptor, encoding gene and recombinant expression vector
ActiveCN105777911AImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSequence signalSingle-Chain Antibodies
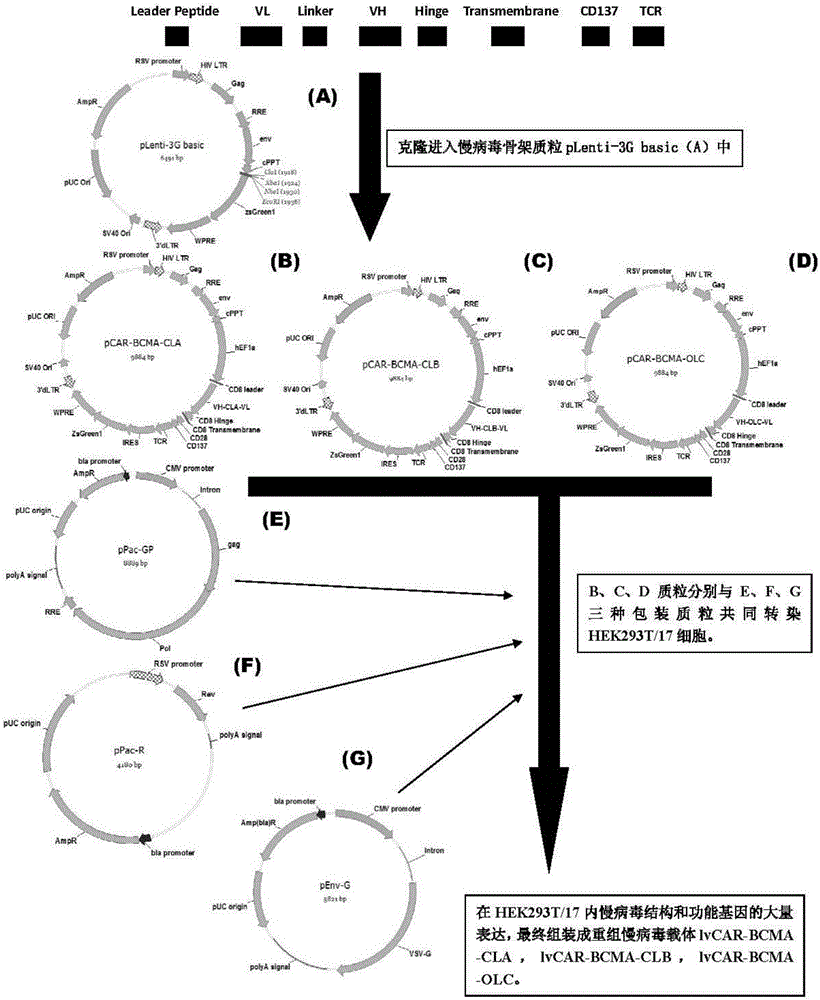
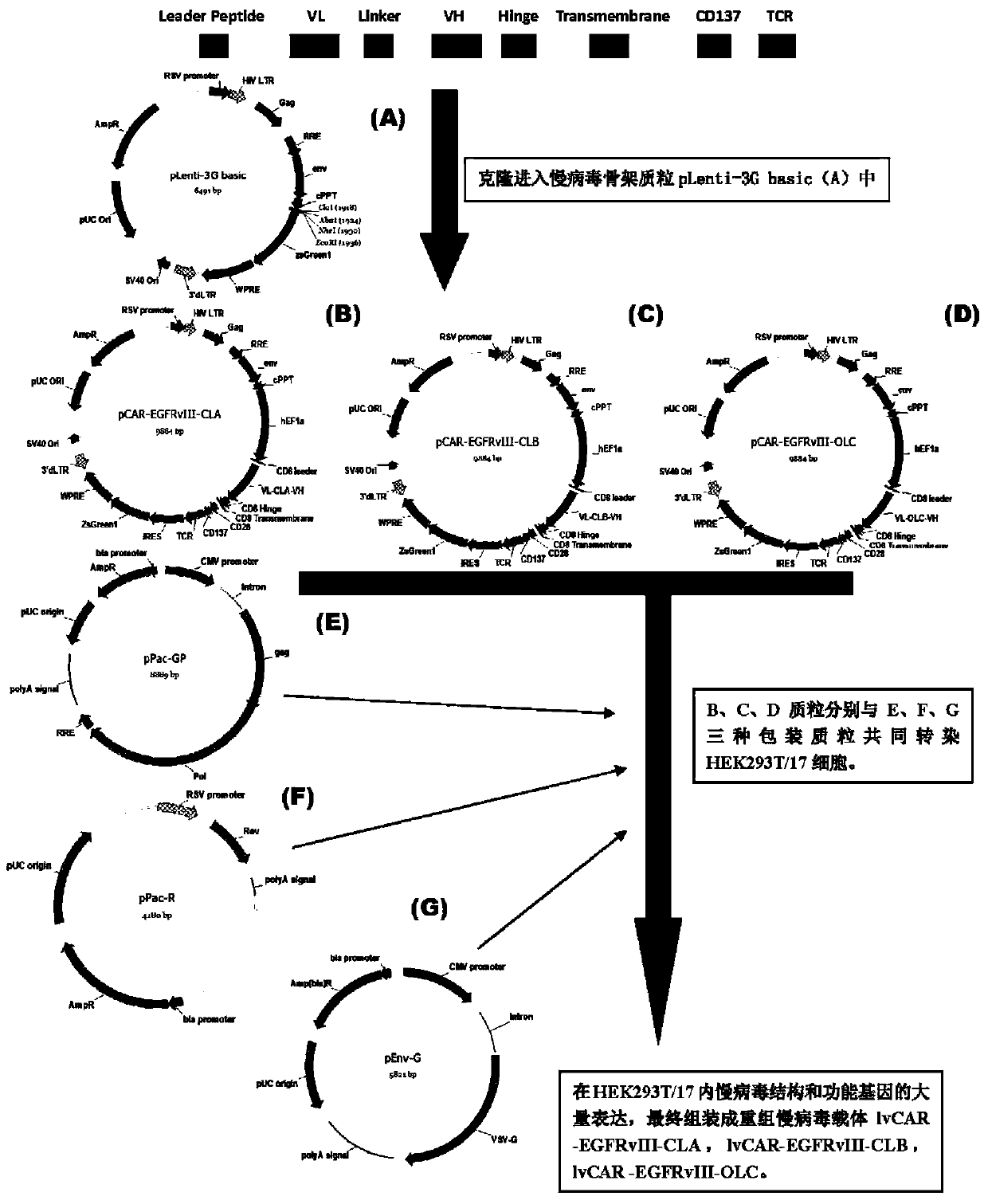
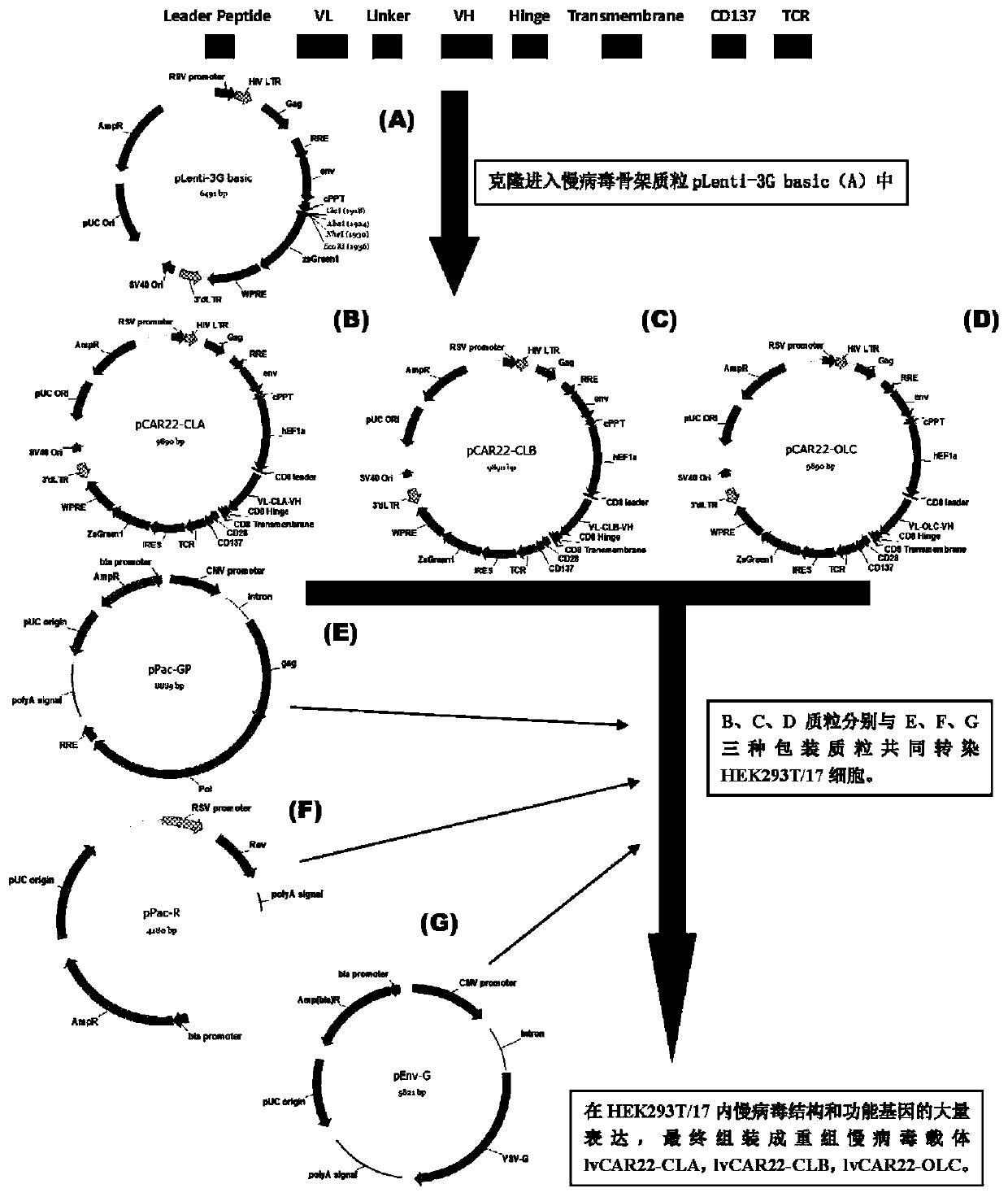
The invention discloses an anti-BCMA chimeric antigen receptor, an encoding gene, a recombinant expression vector and an establishing method and application of the anti-BCMA chimeric antigen receptor, the encoding gene and the recombinant expression vector. The receptor comprises a CD8 leader chimeric receptor signal peptide, a BCMA single-chain antibody heavy chain VH, an Optimal Linker C, a BCMA single-chain antibody light chain VL, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulatory factor and a TCR chimeric receptor T cell activating domain which are sequentially connected in series. In addition, the invention further discloses the encoding gene and the recombinant expression vector of the anti-BCMA chimeric antigen receptor and the establishing method and application of the encoding gene and the recombinant expression vector. The secretion of cell factors and the cytotoxicity in vitro of CAR-T cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Anti-CD20 chimeric antigen receptor, encoding gene, recombinant expression vector, construction method of recombinant expression vector, and application
ActiveCN105949317AImprove in vitro killing effectGood clinical effectMammal material medical ingredientsImmunoglobulinsCD20Single-Chain Antibodies
The invention discloses an anti-CD20 chimeric antigen receptor, an encoding gene, a recombinant expression vector, a construction method of the recombinant expression vector and an application. The anti-CD20 chimeric antigen receptor comprises CD8 leader chimeric receptor signal peptide, CD20 VL, Optimal Linker C, a CD20 single-chain antibody heavy chain VH, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulated factor and a TCR chimeric receptor T cell activating domain which are connected in series. Moreover, the invention discloses an encoding gene of the anti-CD20 chimeric antigen receptor, a recombinant expression vector, a construction method of the recombinant expression vector, and an application. Secretion of cell factors and an in-vitro killing effect of CAR-T cells are obviously improved, and the CAR-T cells have an outstanding clinical treatment effect.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Anti-EGFRvIII chimeric antigen receptor, encoding gene, recombinant expression vector, construction method of recombinant expression vector, and application
ActiveCN105949316AImprove in vitro killing effectGood clinical effectAntibody mimetics/scaffoldsMammal material medical ingredientsSingle-Chain AntibodiesAntigen receptors
The invention discloses an anti-EGFRvIII chimeric antigen receptor, an encoding gene, a recombinant expression vector, a construction method of the recombinant expression vector, and an application. The anti-EGFRvIII chimeric antigen receptor comprises CD8 leader chimeric receptor signal peptide, an EGFRvIII single-chain antibody light chain VL, Optimal Linker C, an EGFRvIII single-chain antibody heavy chain VH, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulated factor and a TCR chimeric receptor T cell activating domain which are connected in series. Moreover, the invention discloses an encoding gene of the anti-EGFRvIII chimeric antigen receptor, a recombinant expression vector, a construction method of the recombinant expression vector, and an application. Secretion of cell factors and an in-vitro killing effect of CAR-T cells are obviously improved, and the CAR-T cells have an outstanding clinical treatment effect.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
CD 123-targeting replication-defective recombinant lentivirus CAR-T transgenic vector as well as construction method and applications thereof
ActiveCN105950664APromote secretionImprove in vitro killing effectMammal material medical ingredientsNucleic acid vectorAntigenFluorescence
The invention discloses a CD 123-targeting replication-defective recombinant lentivirus CAR-T transgenic vector. The CD 123-targeting replication-defective recombinant lentivirus CAR-T transgenic vector comprises a prokaryotic replicor pUC Ori sequence used for plasmid duplication; an amicillin resistance gene AmpR sequence used for amplification of a large number of target strains; a virus replicor SV40 Ori sequence used for enhancing the replication in eukaryocytes; a lentivirus packaging cis element used for lentivirus packaging; ZsGreen 1 green fluorescent protein used for green fluorescence expression of eukaryocytes; an IRES ribosome binding sequence used for co-transcription and co-expression of protein; a human EF1 alpha promoter used for eukaryotic transcription of genes of a chimeric antigen acceptor; the chimeric antigen acceptor used for forming second-generation CAR or third-generation CAR integrating recognition, delivery and promoting; an eWPRE element used for enhancing the expression efficiency of transgenes. In addition, the invention further discloses a construction method and applications of the vector. With the vector, the secretion of the cell factors and the in-vitro lethal effect of the CAR-T cells can be obviously improved, and the effect in treatingacute myelogenous leukemia (AML) clinically is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Anti-HER2 chimeric antigen receptor, encoding gene, recombinant expression vector, construction method of recombinant expression vector, and application
ActiveCN105949318AImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsMammal material medical ingredientsSingle-Chain AntibodiesAntigen receptors
The invention discloses an anti-HER2 chimeric antigen receptor, an encoding gene, a recombinant expression vector, a construction method of the recombinant expression vector, and an application. The anti-HER2 chimeric antigen receptor comprises CD8 leader chimeric receptor signal peptide, an HER2 single-chain antibody heavy chain VH, Optimal Linker C, an HER2 single-chain antibody light chain VL, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulated factor and a TCR chimeric receptor T cell activating domain which are connected in series. Moreover, the invention discloses an encoding gene of the anti-HER2 chimeric antigen receptor, a recombinant expression vector, a construction method of the recombinant expression vector, and an application. Secretion of cell factors and an in-vitro killing effect of CAR-T cells are obviously improved, and the CAR-T cells have an outstanding clinical treatment effect.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
CD22-taregted replication-defective recombinant lentivirus CAR-T transgenic vector as well as construction method and application thereof
ActiveCN105950662APromote secretionImprove in vitro killing effectMammal material medical ingredientsNucleic acid vectorTransgenesisProtein protein
The invention discloses a CD22-taregted replication-defective recombinant lentivirus CAR-T transgenic vector. The vector comprises a prokaryotic replicon pUC Ori sequence for plasmid replication, an ampicillin-containing resistance gene AmpR sequence for mass amplification of a target strain, a virus replicon SV400ri sequence for enhancing replication within eukaryotic cells, a lentivirus packaging cis element for lentivirus packaging, ZsGreen1 green fluorescence protein for expression of green fluorescence of the eukaryotic cells, an IRES ribosome binding sequence for common transcriptional expression of the protein, a human EF1[alpha] promoter for eukaryotic transcription of a chimeric antigen receptor gene, a chimeric antigen receptor for constituting the second or the third generation of CAR integrating identification, transferring and promoting, and an eWPRE element for enhancing a transgenic expression efficiency. In addition, the invention also discloses a construction method and an application of the vector. The vector disclosed by the invention can significantly improve secretion of cell factors and an in vitro killing effect of CAR-T cells; and the vector is obvious in effect on the clinical treatment of precursor B-cell acute lymphocytic leukemia.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
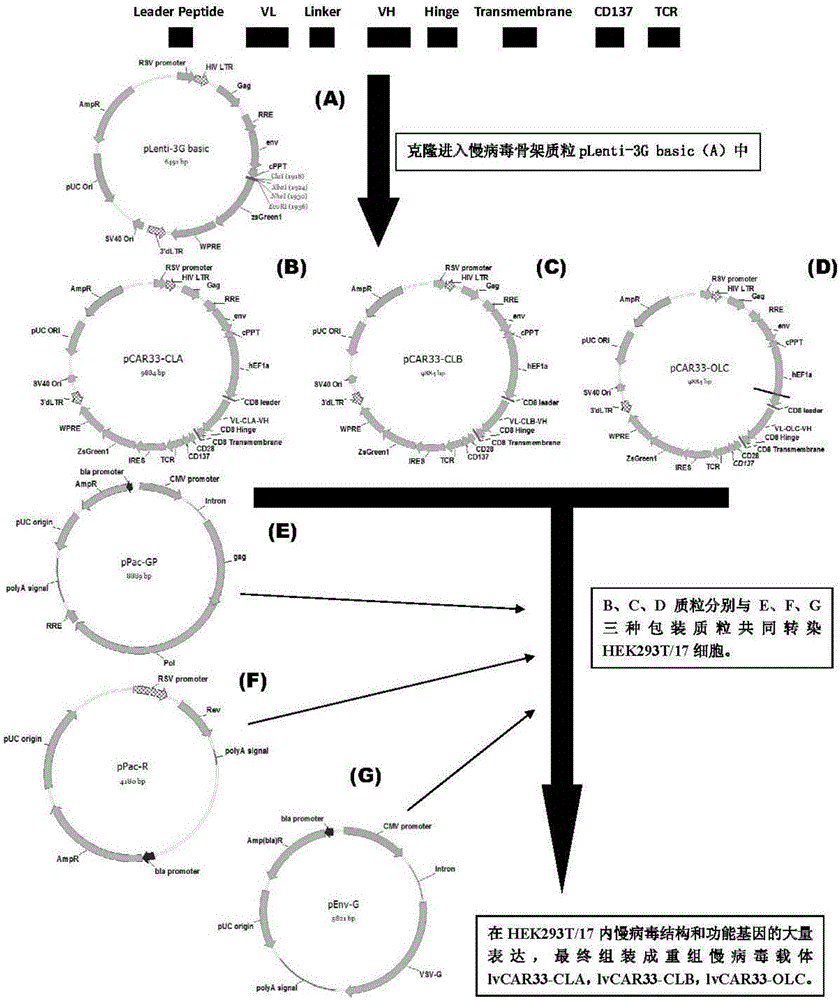
Anti-CD33 chimeric antigen receptor, coding gene, recombinant expression vector and construction method and application of recombinant expression vector
ActiveCN105820255AImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsMammal material medical ingredientsSingle-Chain AntibodiesCD33
The invention discloses an anti-CD33 chimeric antigen receptor, a coding gene, a recombinant expression vector and a construction method and an application of the recombinant expression vector. The anti-CD33 chimeric antigen receptor comprises a CD8leader chimeric receptor signal peptide, a heavy chain VL of a CD33 single-chain antibody, an Optimal Linker C, a light chain VH of the CD33 single-chain antibody, a CD8Hinge chimeric receptor hinge, a CD8Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulation factor and a TCR chimeric receptor T-cell activation domain, which are serially connected in sequence. In addition, the invention discloses the coding gene of the anti-CD33 chimeric antigen receptor, the recombinant expression vector and the construction method and the application of the recombinant expression vector. According to the anti-CD33 chimeric antigen receptor, the secretion of cell factors and the in vitro killing effect of CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
CD 30-targeting replication-defective recombinant lentivirus CAR-T transgenic vector as well as construction method and applications thereof
ActiveCN105950663APromote secretionImprove in vitro killing effectMammal material medical ingredientsNucleic acid vectorAntigenAmpicillin
The invention discloses a CD 30-targeting replication-defective recombinant lentivirus CAR-T transgenic vector. The CD 30-targeting replication-defective recombinant lentivirus CAR-T transgenic vector comprises a prokaryotic replicor pUC Ori sequence used for plasmid duplication; an ampicillin resistance gene AmpR sequence used for amplification of a large number of target strains; a virus replicor SV40 Ori sequence used for enhancing the replication in eukaryocytes; a lentivirus packaging cis element used for lentivirus packaging; ZsGreen 1 green fluorescent protein used for green fluorescence expression of the eukaryocytes; an IRES ribosome binding sequence used for co-transcription and co-expression of protein; a human EF1 alpha promoter used for eukaryotic transcription of genes of a chimeric antigen acceptor; the chimeric antigen acceptor used for forming second-generation CAR or third-generation CAR integrating recognition, delivery and promoting; and an eWPRE element used for enhancing the expression efficiency of transgenes. In addition, the invention further discloses a construction method and applications of the vector. With the vector, the secretion of the cell factors and the in-vitro lethal effect of the CAR-T cells can be obviously improved, and the effect in treating hodgkin lymphoma or non-hodgkin lymphoma clinically is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
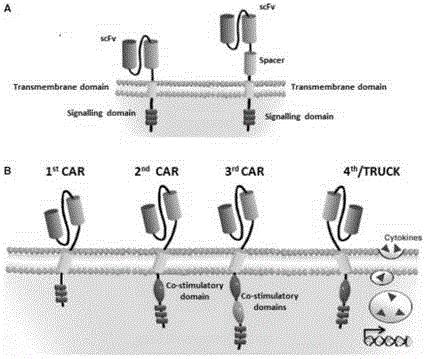
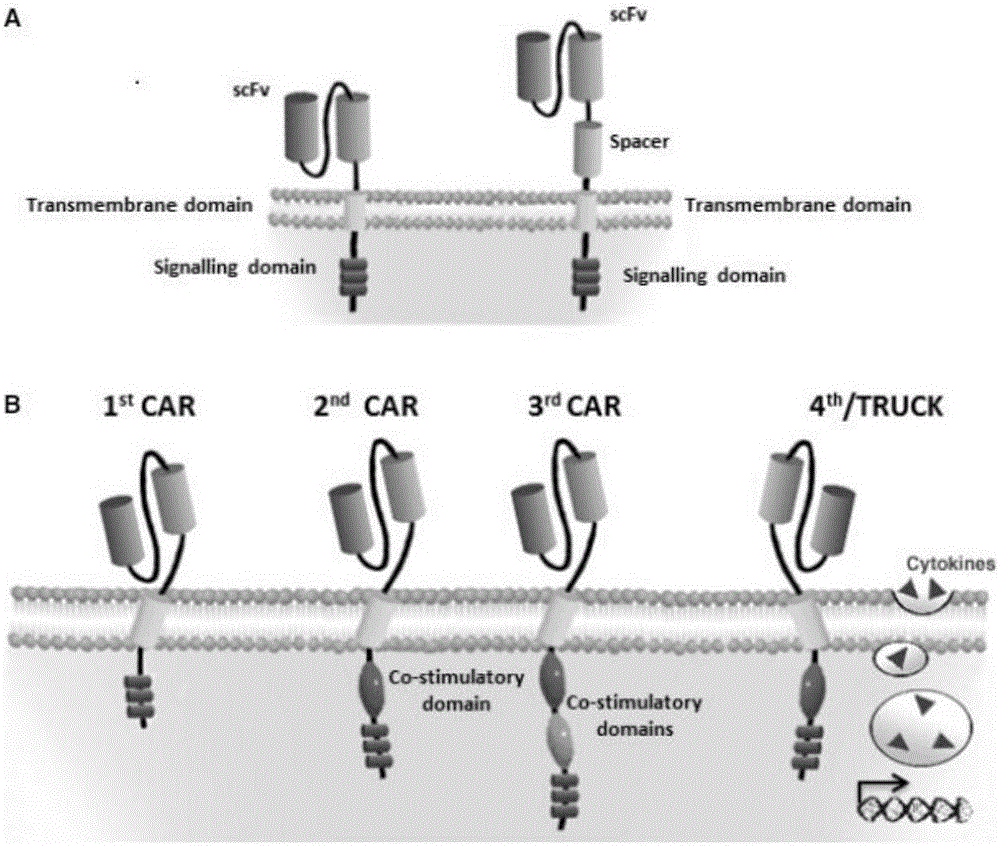
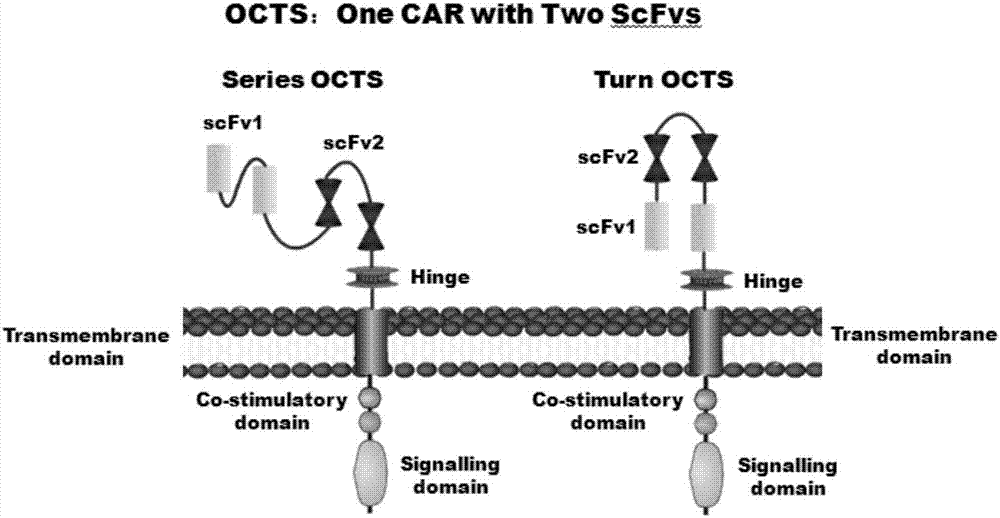
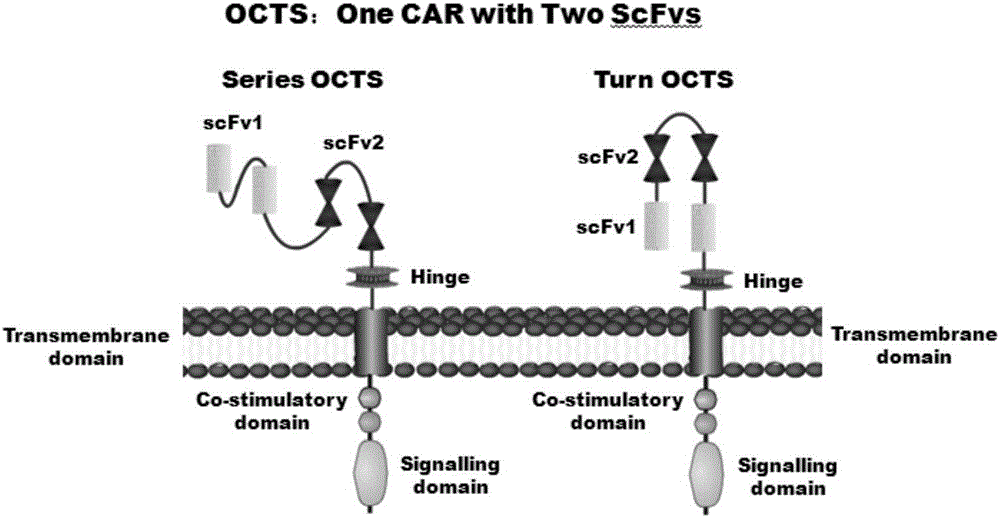
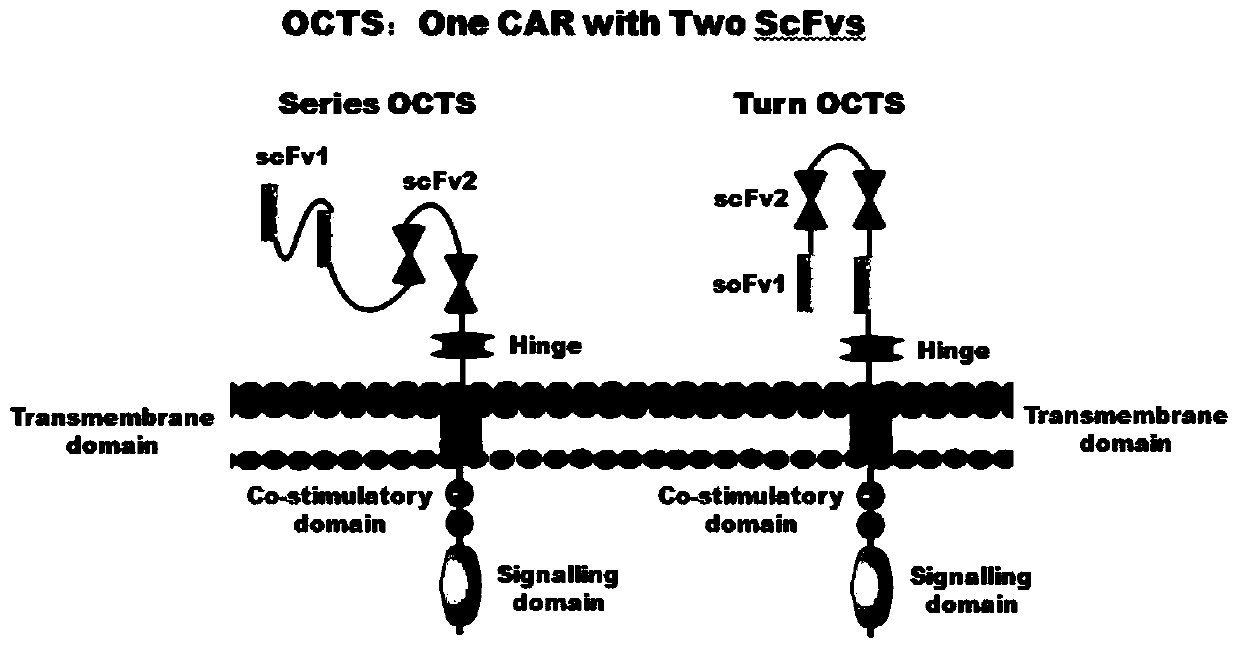
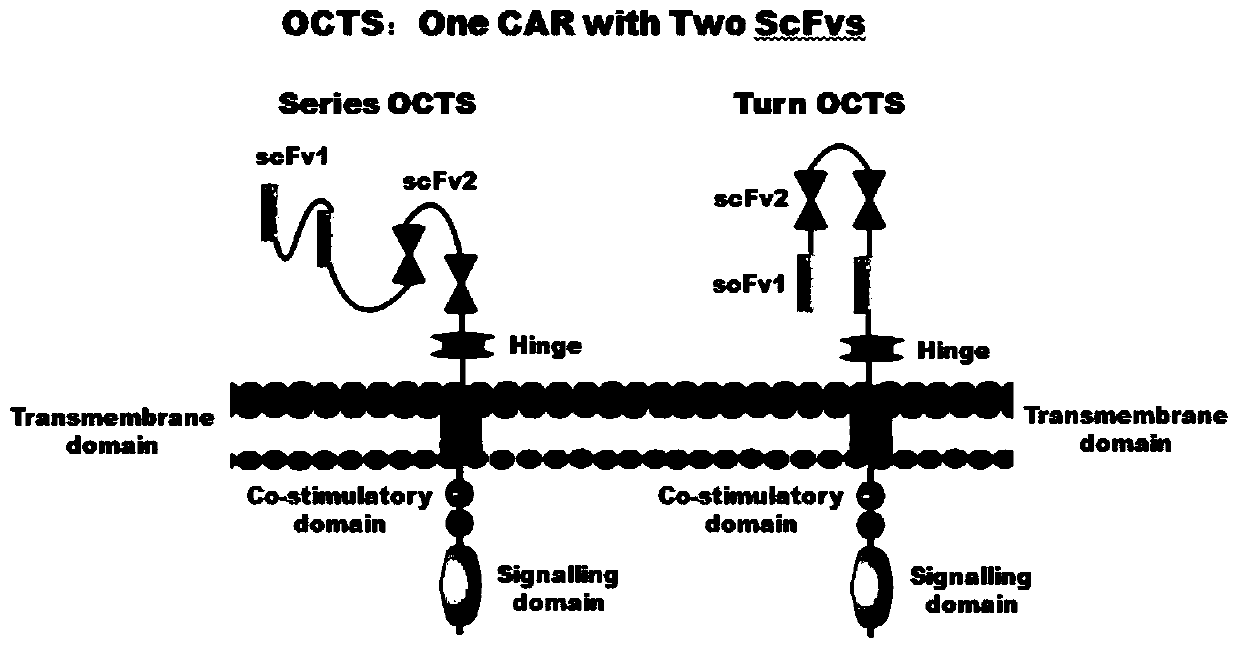
Lymphoblastic leukemia CAR-T (Chimeric Antigen Receptor-T Cell Immunotherapy) therapy carrier based on OCTS (One CAR with Two SeFvs) technology as well as constructing method and application of lymphoblastic leukemia CAR-T therapy carrier
ActiveCN107245500AExpand the scope of recognitionAvoid batch cultureMammal material medical ingredientsNGF-receptor/TNF-receptor superfamilySequence signalCD20
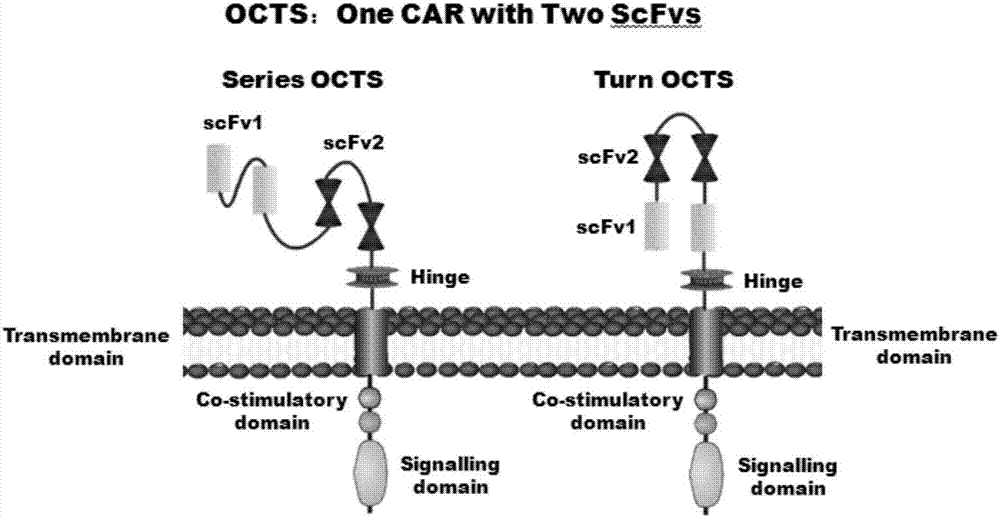
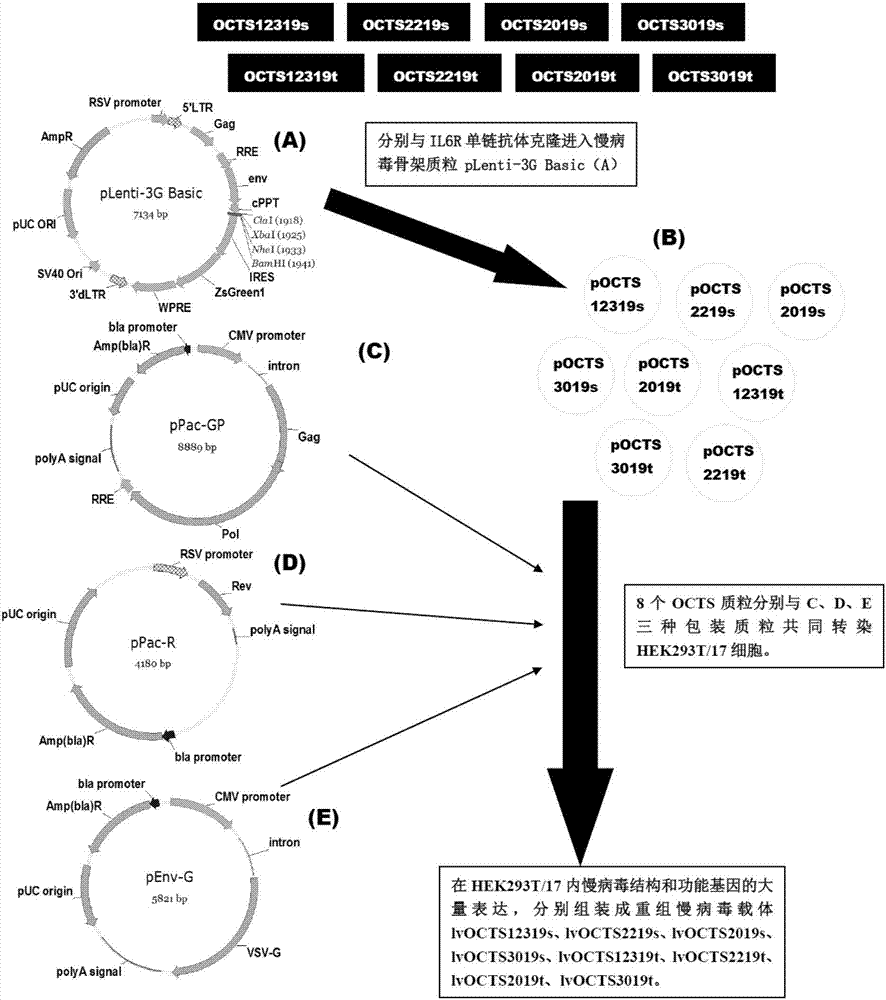
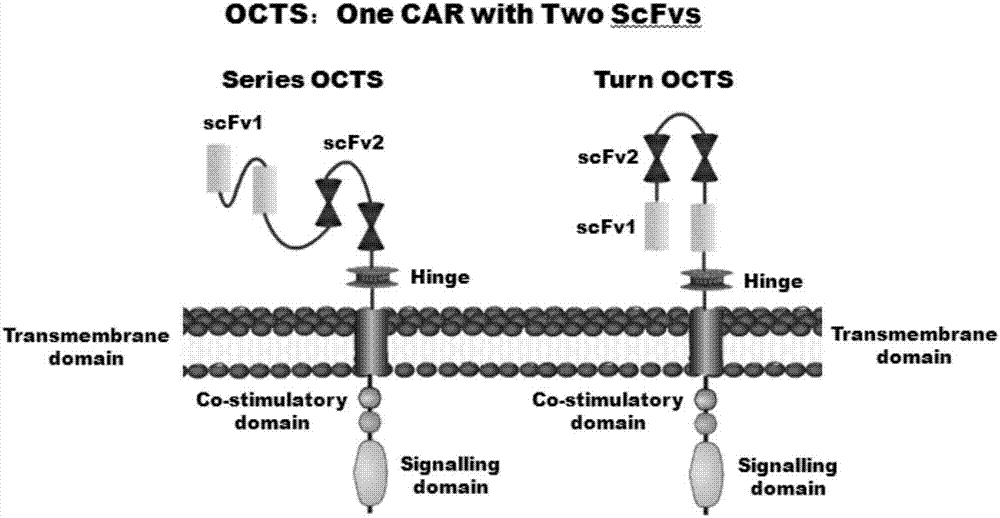
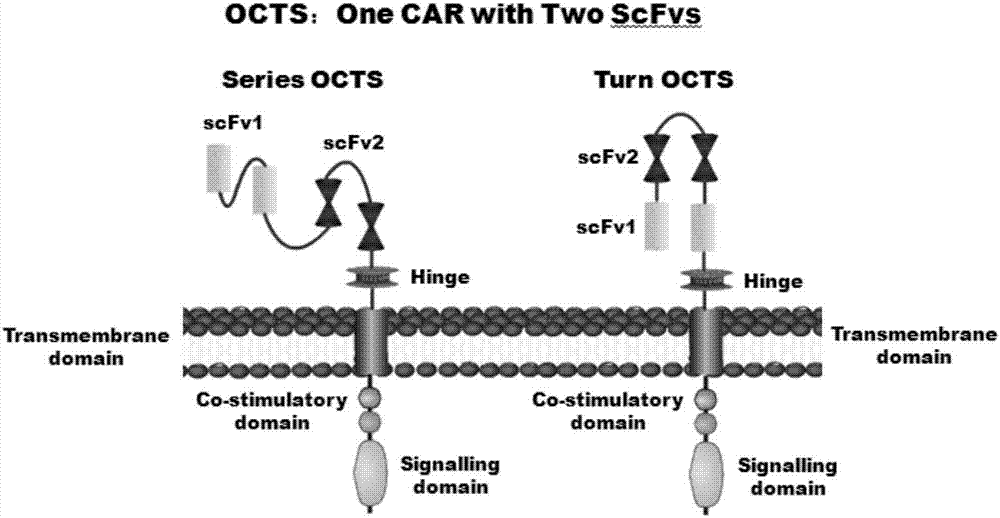
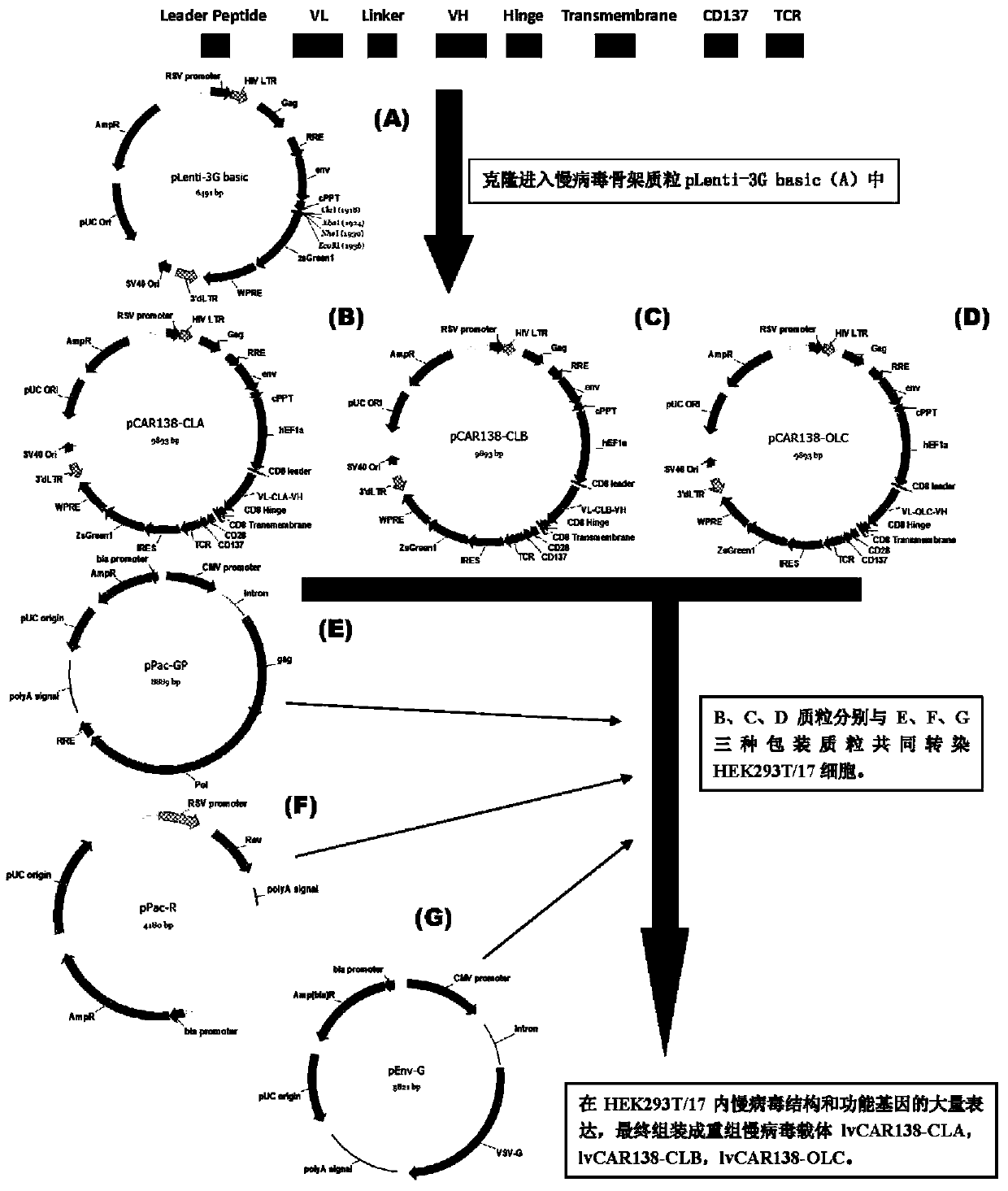
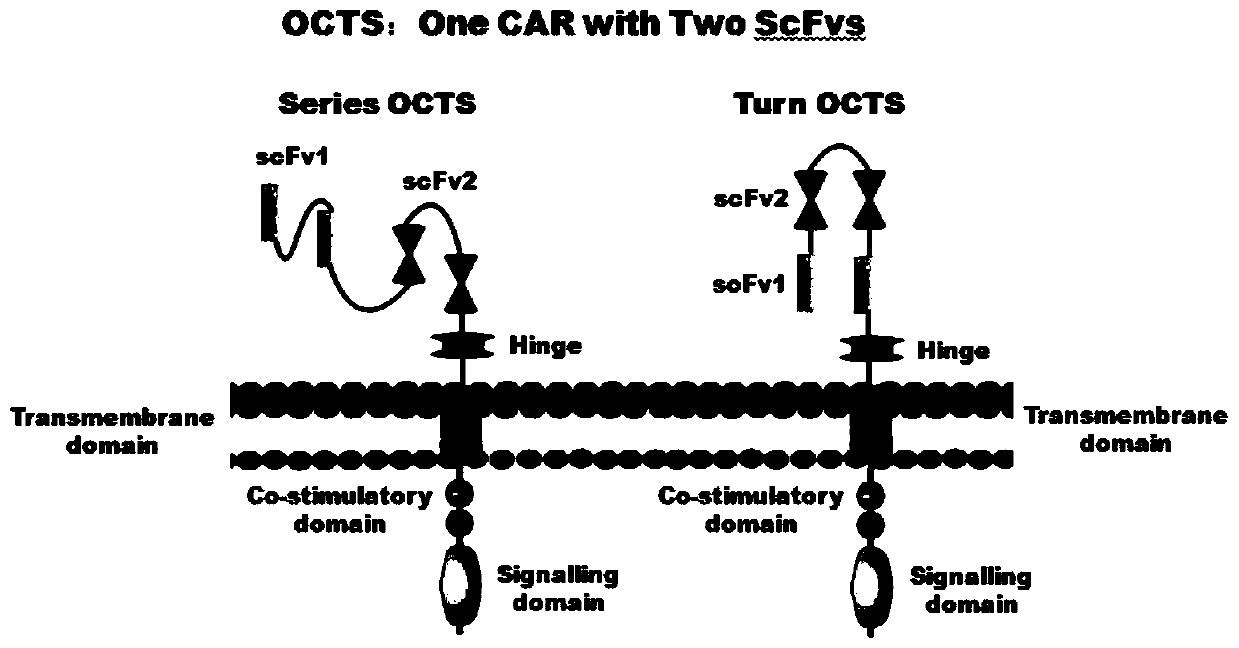
The invention discloses a lymphoblastic leukemia CAR-T (Chimeric Antigen Receptor-T Cell Immunotherapy) therapy carrier based on an OCTS (One CAR with Two SeFvs) technology. The lymphoblastic leukemia CAR-T therapy carrier comprises a lentivirus skeleton plasma, a human EF1alpha promoter, an OCTS chimeric receptor structural domain and an IL6R single-chain antibody, wherein the OCTS chimeric receptor structural domain comprises a CD8 leader chimeric receptor signal peptide and two groups of single-chain antibodies; the first group of single-chain antibodies is selected from any one of the following four groups of single-chain antibodies: a CD20 single-chain antibody light chain VL and a CD20 single-chain antibody heavy chain VH, a CD22 single-chain antibody light chain VL and a CD22 single-chain antibody heavy chain VH, a CD30 single-chain antibody light chain VL and a CD30 single-chain antibody heavy chain VH, and a CD123 single-chain antibody light chain VL and a CD123 single-chain antibody heavy chain VH; and the second group of the single-chain antibodies is a CD19 single-chain antibody light chain VL and a CD19 single-chain antibody heavy chain VH, an antibody Inner-Linker, a single-chain antibody Inter-Linker, a CD8-Hinge chimeric receptor linker, a CD8 Transmembrane chimeric receptor transmembrane zone, a TCR (T Cell Receptor) chimeric receptor T cell activation domain and a chimeric receptor co-stimulator zone. Besides, the invention discloses a constructing method for the carrier and application of the carrier to preparation of a drug for treating lymphoblastic leukemia.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
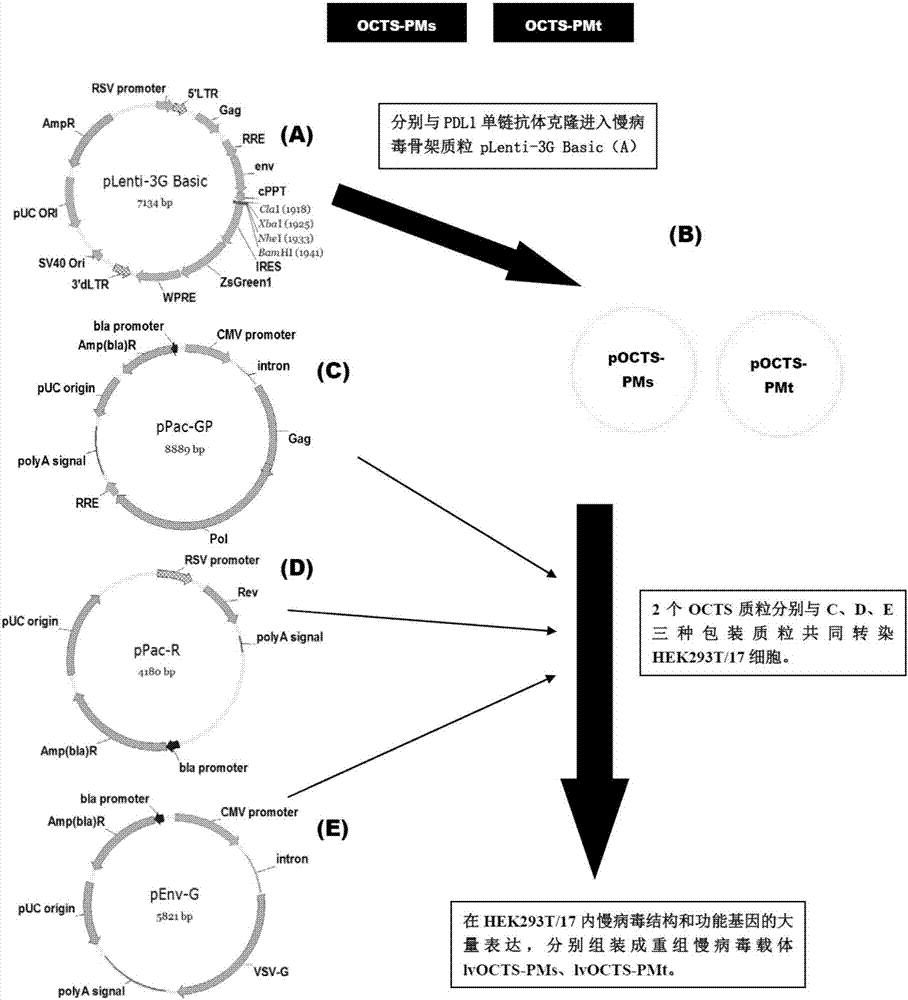
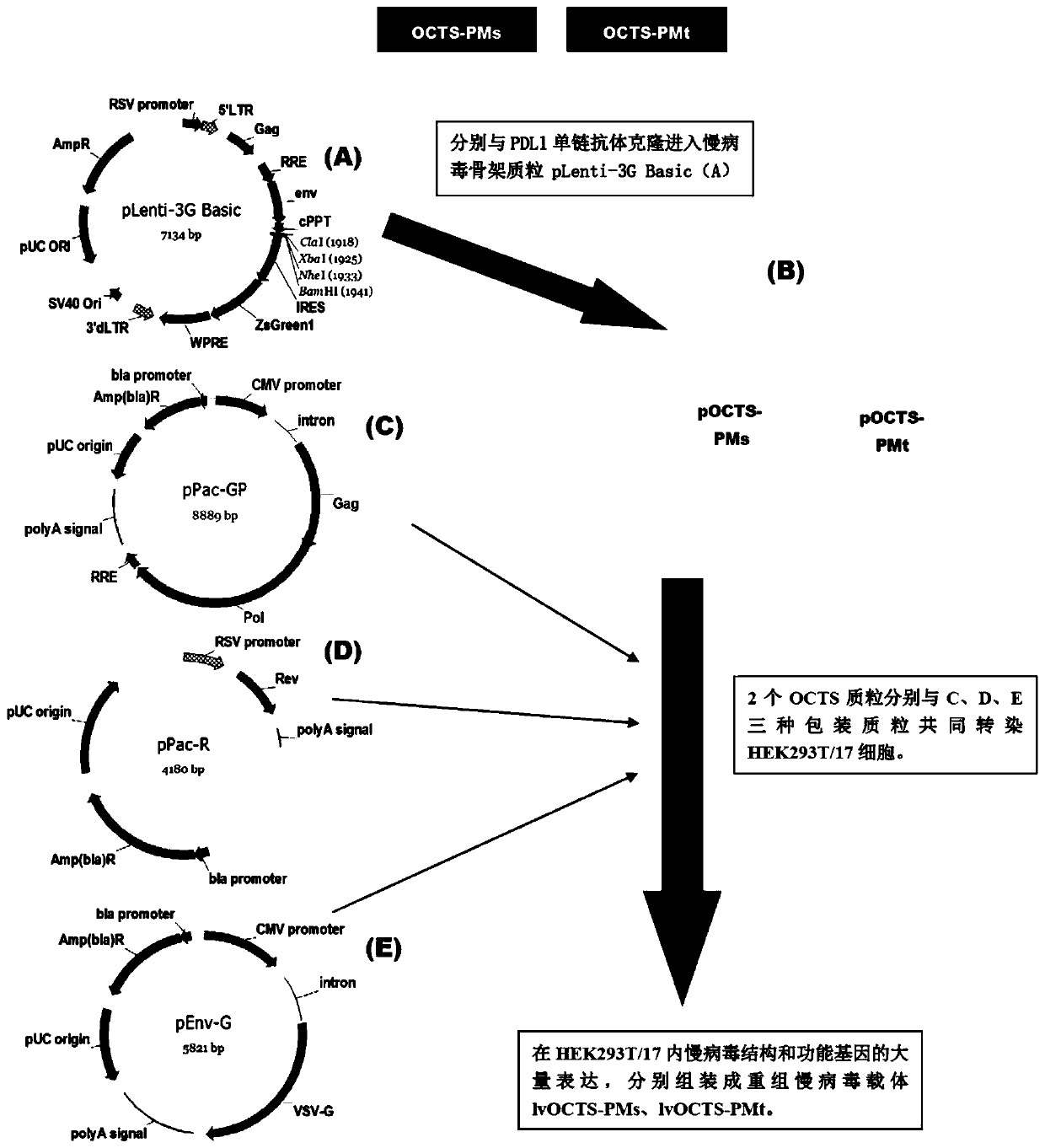
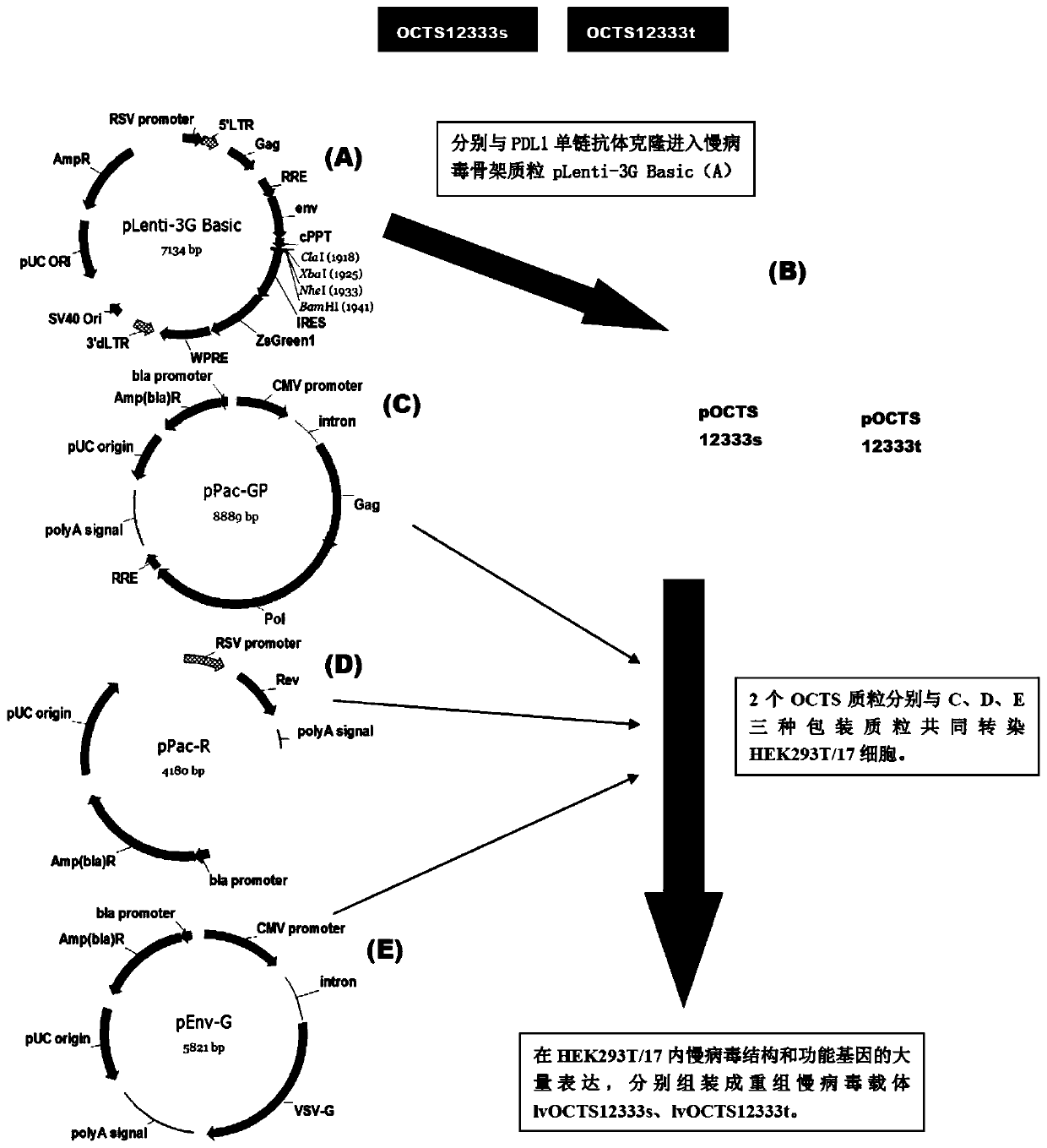
OCTS (One CAR (Chimeric Antigen Receptor) with two ScFvs (Single-chain variable Fragments)) technique based CAR-T (Chimeric Antigen Receptor-T cell immunotherapy) therapeutic vector for glioblastoma and construction method and application thereof
ActiveCN107267555ADirect and activate killingActivate killingVirusesPeptide/protein ingredientsSingle-Chain AntibodiesEukaryotic plasmids
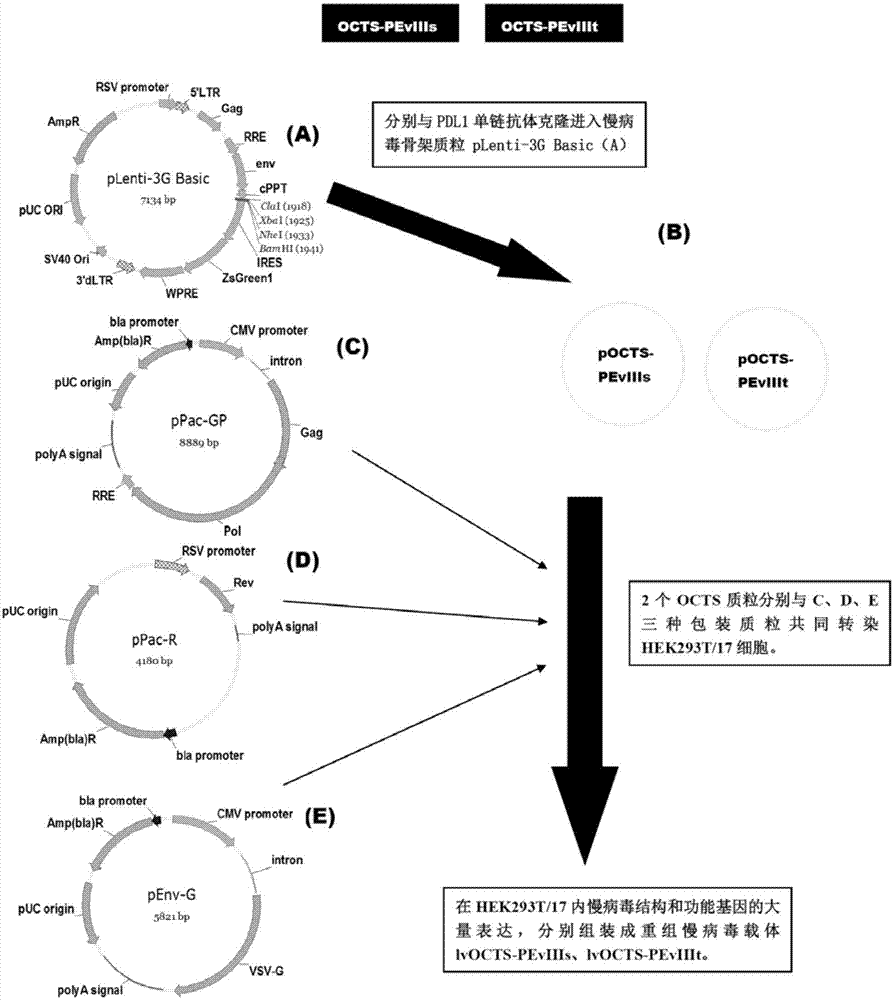
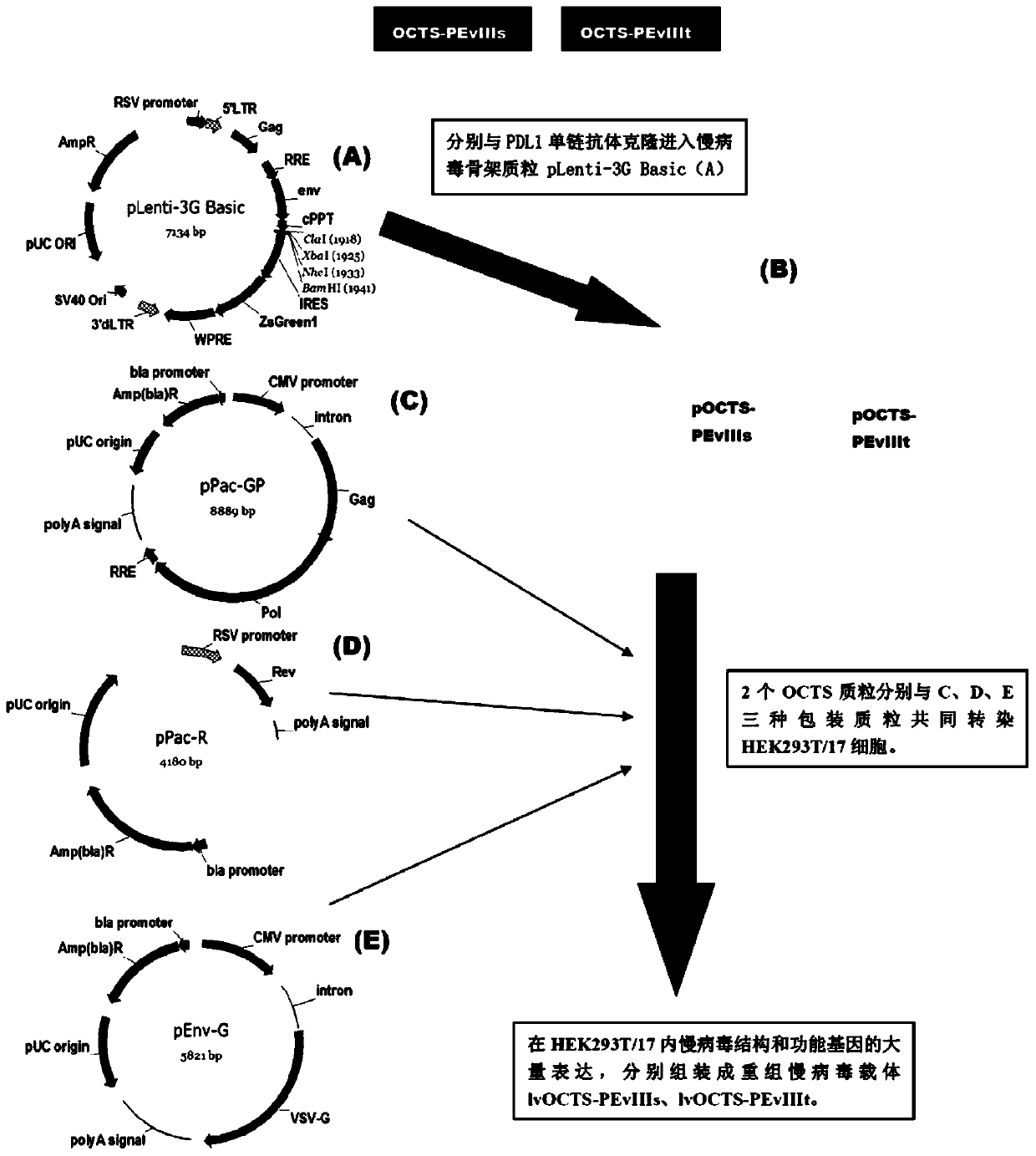
The invention discloses an OCTS (One CAR (Chimeric Antigen Receptor) with two ScFvs (Single-chain variable Fragments)) technique based CAR-T (Chimeric Antigen Receptor-T cell immunotherapy) therapeutic vector for glioblastoma. The OCTS technique based CAR-T therapeutic vector comprises a lentiviral skeleton plasmid, a human EF1 [alpha] promoter (SEQ ID NO. 14), an OCTS chimeric receptor structural domain and a PDL1 single-chain antibody, wherein the OCTS chimeric receptor structural domain comprises a CD8 leader chimeric receptor signal peptide (SEQ ID NO. 15), a PDL1 single-chain antibody light chain VL (SEQ ID NO. 16), a PDL1 single-chain antibody heavy chain VH (SEQ ID NO. 17), an EGFRvIII single-chain antibody light chain VL (SEQ ID NO. 18), an EGFRvIII single-chain antibody heavy chain VH (SEQ ID NO. 19), an antibody Inner-Linker (SEQ ID NO. 20), a single-chain antibody Inter-Linker (SEQ ID NO. 21), a CD8 Hinge chimeric receptor linker (SEQ ID NO. 22), a CD8 Transmembrane chimeric receptor transmembrane domain (SEQ ID NO. 23), a TCR (T Cell Receptor) chimeric receptor T cell activation domain (SEQ ID NO. 26) and a chimeric receptor co-stimulator domain. In addition, the invention also discloses a construction method of the vector and application of the vector to the preparation of a medicine for treating the glioblastoma.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
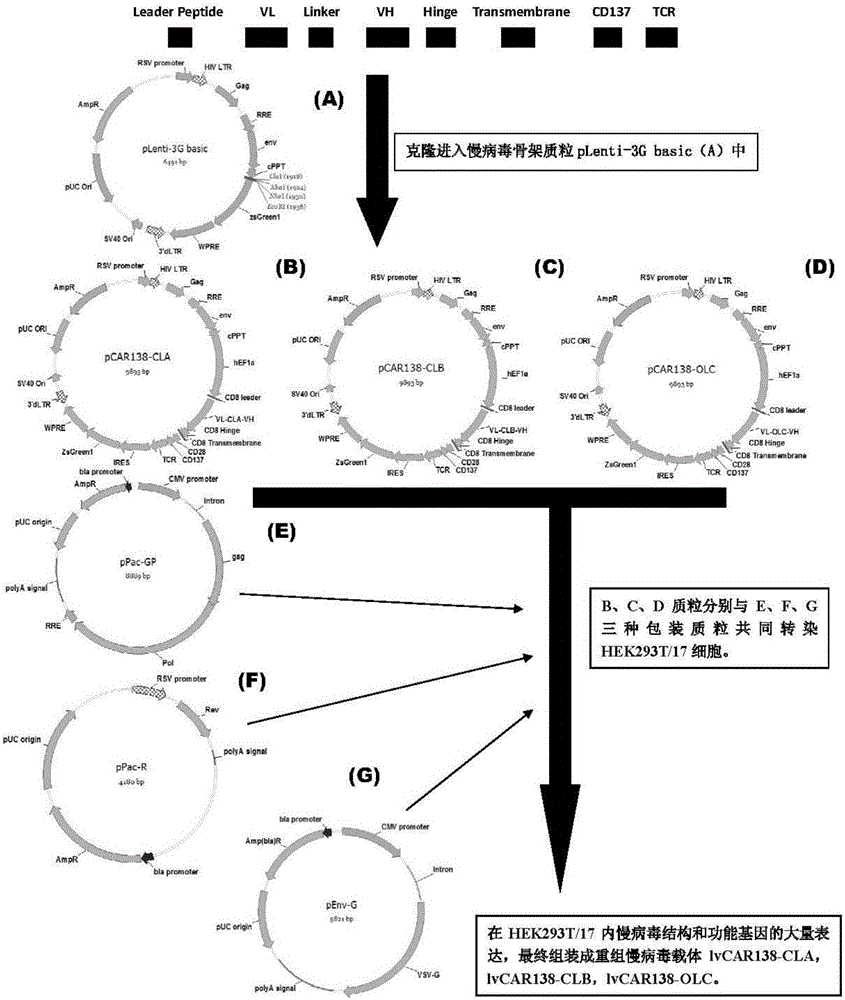
Anti-CD138 chimeric antigen receptor, coding gene, recombinant expression vector and construction method and application of recombinant expression vector
ActiveCN105820254AImprove in vitro killing effectGood clinical effectVirusesAntibody mimetics/scaffoldsSequence signalSingle-Chain Antibodies
The invention discloses an anti-CD138 chimeric antigen receptor, a coding gene, a recombinant expression vector and a construction method and an application of the recombinant expression vector. The anti-CD138 chimeric antigen receptor comprises a CD8leader chimeric receptor signal peptide, a light chain VH of the CD138 single-chain antibody, an Optimal Linker C, a heavy chain VL of a CD138 single-chain antibody, a CD8Hinge chimeric receptor hinge, a CD8Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulation factor and a TCR chimeric receptor T-cell activation domain, which are serially connected in sequence. In addition, the invention discloses the coding gene of the anti-CD138 chimeric antigen receptor, the recombinant expression vector and the construction method and the application of the recombinant expression vector. According to the anti-CD138 chimeric antigen receptor, the secretion of cell factors and the in vitro killing effect of CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Mesothelin-targeted replication-defective recombinant lentivirus CAR-T transgenic carrier as well as establishment method and application thereof
ActiveCN105969805APromote secretionImprove in vitro killing effectMammal material medical ingredientsNucleic acid vectorEucaryotic cellFluorescence
The invention discloses a Mesothelin-targeted replication-defective recombinant lentivirus CAR-T transgenic carrier which comprises a pronucleus replicon pUC Ori sequence for plasmid replication, an amicillin resistance gene AmpR containing sequence for mass amplification of target strain, virus replicon SV40Ori sequence for enhancing replication in eukaryocyte, a lentivirus packaging cis-element for lentivirus packaging, a ZsGreen1 green fluorescent protein for green fluorescence expression of eukaryocyte, an IRES ribosome combination sequence for joint transcriptional expression of protein, a human EF1(alpha) promoter for the eukaryotic transcription of chimeric antigen receptor gene, a chimeric antigen receptor for forming a second-generation CAR or third-generation CAR integrating identification, transfer and start, and an eWPRE element for improving the transgenic expression efficiency. Moreover, the invention also discloses an establishment method and application of the carrier. In the invention, the secretion of cell factors and the in-vitro killing effect of CAR-T cells can be remarkably enhanced, and the effect of clinical treatment of malignant pleural mesothelioma and pancreatic cancer is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Marker used for in-vivo tracing and manual removal of CAR-T cells and application thereof
ActiveCN107287207AActivate killingGuaranteed normal treatmentMammal material medical ingredientsImmunoglobulinsCAR T-cell therapyT lymphocyte
The invention discloses a marker used for in-vivo tracing and manual removal of CAR-T cells. The marker comprises a trace-linker 1 with a sequence as shown in SEQ ID No. 18, a trace-linker 2 with a sequence as shown in SEQ ID No. 19 and a trace-linker 3 with a sequence as shown in SEQ ID No. 19. The invention also discloses a CAR-T therapy vector including the marker and a construction method thereof. Moreover, the invention further discloses application of the marker to preparation of the CAR-T therapy vector and drugs used for treating triple-negative breast cancer. The marker provided by the invention can realize controllable in-vivo tracing, in-vitro separation and manual removal of CAR-T cells without influence on the tumor killing effect of the CAR-T cells, so the security of CAR-T cell therapy is greatly improved, and a powerful pool can be provided for deeper analysis of the process of CAR-T cell therapy. The vector provided by the invention can express a targeting chimeric antigen receptor of CD117 in human T lymphocytes, guides and activates killing effect of T lymphocytes on CD117 positive cells, and is applicable to treatment of triple-negative breast cancer in clinical practice.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
OCTS technology-based pancreatic cancer and malignant mesothelioma CAR-T therapy vector, as well as construction method and applicaitno thereof
ActiveCN107299110AExpand the scope of recognitionAvoid batch cultureVirusesPeptide/protein ingredientsSingle-Chain AntibodiesCD8
The invention discloses an OCTS technology-based pancreatic cancer and malignant mesothelioma CAR-T therapy vector. The vector comprises lentivirus framework plasmid, a human EF1alpha promoter (SEQ ID NO. 14), an OCTS chimeric receptor structural domain and a PDL1 single-chain antibody; the OCTS chimeric receptor structural domain comprises CD8 leader chimeric receptor signal peptide (SEQ ID NO. 15), a PDL1 single-chain antibody light chain VL (SEQ ID NO. 16), a PDL1 single-chain antibody heavy chain VH (SEQ ID NO. 17), an MESOTHELIN single-chain antibody light chain VL (SEQ ID NO. 18), an MESOTHELIN single-chain antibody heavy chain VH (SEQ ID NO. 19), an antibody inner linker Inner-Linker (SEQ ID NO. 20), a single-chain antibody inter-linker Inter-Linker (SEQ ID NO. 21), a CD8 Hinge chimeric receptor linker (SEQ ID NO. 22), a CD8Transmembrane chimeric receptor transmembrane domain (SEQ ID NO. 23), a TCR chimeric receptor T-cell activation domain (SEQ ID NO. 26), and a chimeric receptor inducible co-stimulater area. In addition, the invention also discloses a construction method of the vector as well as application of the vector to preparation of medicines for treating pancreatic cancer and malignant mesothelioma.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
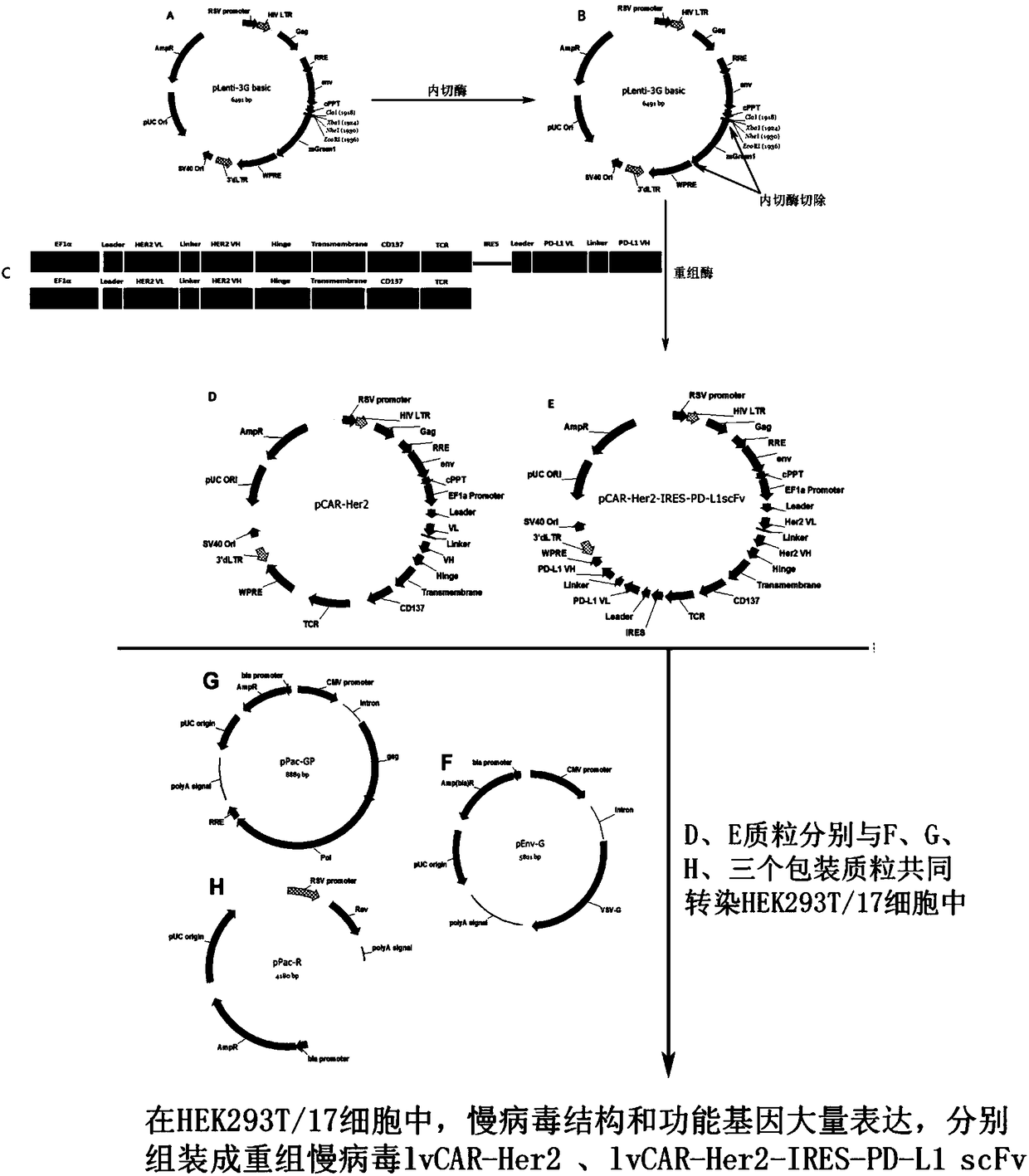
CAR-T vector capable of targeting Her2 and blocking PD-L1 to reduce tumor immune escape and construction method and use thereof
InactiveCN108203720AIncrease lethalityReduce immune escapeAntibody mimetics/scaffoldsMammal material medical ingredientsSingle-Chain AntibodiesT lymphocyte
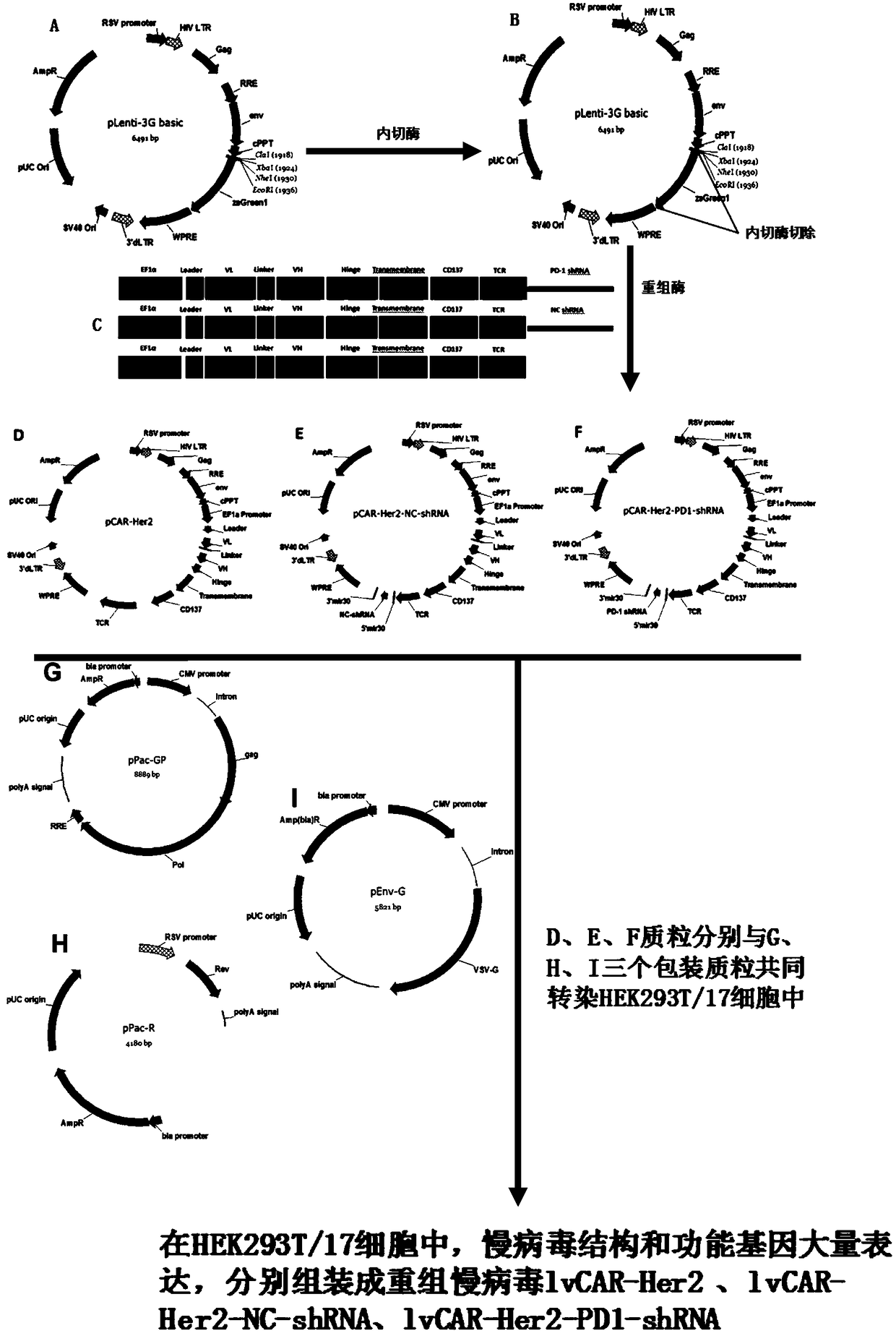
The invention discloses a CAR-T vector capable of targeting Her2 and blocking PD-L1 to reduce tumor immune escape. The CAR-T vector comprises a human EF1 alpha promoter for eukaryotic transcription ofa chimeric antigen receptor gene, wherein the human EF1 alpha promoter is shown in the formula of SEQ ID NO. 1, a second-generation CAR or third-generation CAR chimeric antigen receptor combining recognition, delivery and starting, wherein the chimeric antigen receptor comprises a Her2 single-chain antibody light chain VL and a Her2 single-chain antibody heavy chain VH, an IRES ribosome binding sequence shown in the formula of SEQ ID NO. 10, PD-L1 scFv and a gene transfer vector. The invention discloses a construction method of the vector and a use of the vector in preparation of drugs for reducing tumor immune escape. Through a Her2 CAR-T technology and PD-L1 scFv expressed in the T lymphocytes, the PD-L1 on the surface of the tumor cells is blocked, the interaction between PD-1 and PD-L1 is inhibited, tumor cell immune escape is inhibited and the T lymphocyte effects of killing tumor cells are improved so that the CAR-T immunotherapy effects of resisting brain glioma are improved.
Owner:上海生博生物医药科技有限公司
CAR-T vector capable of targeting Her2 and disturbing PD-L1 to reduce tumor immune escape and construction method and use thereof
InactiveCN108203717AReduce immune escapeGood curative effectMammal material medical ingredientsAntibody ingredientsGene deliverySingle-Chain Antibodies
The invention discloses a CAR-T vector capable of targeting Her2 and disturbing PD-L1 to reduce tumor immune escape. The CAR-T vector comprises a human EF1 alpha promoter for eukaryotic transcriptionof a chimeric antigen receptor gene, wherein the human EF1 alpha promoter is shown in the formula of SEQ ID NO. 1, PD-1 shRNA in the 3'UTR, wherein the sequence of the PD-1 shRNA is shown in the formula of SEQ ID NO. 25, a gene delivery vector, and a second-generation CAR or third-generation CAR chimeric antigen receptor combining recognition, delivery and starting, wherein the chimeric antigen receptor comprises a Her2 single-chain antibody light chain VL and a Her2 single-chain antibody heavy chain VH. The invention also discloses a construction method of the vector and a use of the vector in preparation of drugs for disturbing PD-L1 to reduce tumor immune escape. Through a Her2 CAR-T technology and interference of PD-1 expression in T lymphocytes, the tumor immune escape is inhibited and the T lymphocyte effects of killing tumor cells are improved so that the CAR-T immunotherapy effects of resisting brain glioma are improved.
Owner:上海生博生物医药科技有限公司
OCTS technology-based prostatic cancer CAR-T therapy vector and construction method and application thereof
ActiveCN107164410AExpand the scope of recognitionAvoid batch cultureMammal material medical ingredientsFermentationSingle-Chain AntibodiesEukaryotic plasmids
The invention discloses an OCTS technology-based prostatic cancer CAR-T therapy vector which comprises a lentivirus framework plasmid, a human EP1alpha promoter (SEQ ID NO.14), an OCTS chimeric receptor structure domain and a PDL1 single-chain antibody, wherein the OCTS chimeric receptor structure domain comprises A CD8 leader chimeric receptor signal peptide (SEQ ID NO.15), a PSMA single-chain antibody light chain VL (SEQ ID NO.16), a PSMA single-chain antibody heavy chain VH (SEQ ID NO.17), a PDL1 single-chain antibody light chain VL (SEQ ID NO.18), a PDL1 single-chain antibody heavy chain VH (SEQ ID NO.19), an antibody Inner-Linker (SEQ ID NO.20), a single-chain antibody Inter-Linker (SEQ ID NO.21), a CD8 Hinge chimeric receptor hinge (SED ID NO.22), a CD8 Transmembrane chimeric receptor transmembrane domain (SEQ ID NO.23), a TCR chimeric receptor T cell activation domain (SEQ ID NO.26) and a chimeric receptor co-stimulus factor domain. In addition, the invention further discloses a construction method of the vector and an application of the vector in preparation of drugs for treating a prostatic cancer.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Myeloid leukemia CAR-T treatment carrier based on OCTS technology and construction method and application of myeloid leukemia CAR-T treatment carrier
ActiveCN107177632AExpand the scope of recognitionAvoid batch cultureMammal material medical ingredientsNGF-receptor/TNF-receptor superfamilySingle-Chain AntibodiesMyeloid leukemia
The invention discloses a myeloid leukemia CAR-T treatment carrier based on an OCTS technology. The myeloid leukemia CAR-T treatment carrier based on the OCTS technology comprises lentivirus framework plasmids, a human EF1 alpha promoter (SEQ ID NO. 14), an OCTS chimeric receptor structural domain and a PDL1 single-chain antibody; the OCTS chimeric receptor structural domain comprises a CD8 leader chimeric receptor signal peptide (SEQ ID NO. 15), a CD33 single-chain antibody light chain VL (SEQ ID NO. 16), a CD33 single-chain antibody heavy chain VH (SEQ ID NO. 17), a CD123 single-chain antibody light chain VL (SEQ ID NO. 18), a CD123 single-chain antibody heavy chain VH (SEQ ID NO. 19), an antibody Inner-Linker (SEQ ID NO. 20), a single-chain antibody Inter-Linker (SEQ ID NO. 21), a CD8 Hinge chimeric receptor hinge (SEQ ID NO. 22), a CD8 Transmembrane chimeric receptor region (SEQ ID NO. 23), a TCR chimeric receptor T cell activation domain (SEQ ID NO. 26) and a chimeric receptor co-stimulatory factor region. In addition, the invention further discloses a construction method of the carrier and application of the carrier in preparation of drugs for treating myeloid leukemia.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
A car-t transgene vector based on replication-deficient recombinant lentivirus and its construction method and application
ActiveCN105602992BPromote secretionImprove in vitro killing effectGenetic material ingredientsFermentationAgricultural scienceAmpicillin
The invention discloses a CAR-T transgene vector based on replication defective recombinant lentivirus. The CAR-T transgene vector comprises an original nuclear replicon pUCOri sequence, a resistance gene AmpR sequence containing ampicillin, a virus replicon SV40 Ori sequence, a lentivirus packaging cis element, ZsGreen1 green fluorescent protein, an IRES ribosome binding sequence, a human EF1 alpha promoter , a chimeric antigen receptor of second-generation CAR or third-generation CAR and a regulating element, wherein the original nuclear replicon pUCOri sequence is used for plasmid replication; the resistance gene AmpR sequence is used for massively proliferating target strains; the virus replicon SV40 Ori sequence is used for enhancing replication in eukaryocyte; the lentivirus packaging cis element is used for lentivirus packaging; the ZsGreen1 green fluorescent protein is used for expressing green fluorescent for eukaryocyte; the IRES ribosome binding sequence is used for jointly transcribing and expressing protein; the human EF1 alpha promoter is used for conducting eukaryotic transcription on antigen receptor genes; the chimeric antigen receptor is used for forming the second-generation CAR or the third-generation CAR integrating recognition, transfer and start; the regulating element is used for enhancing expression efficiency of transgenes and used after eWPRE-enhanced type woodchuck hepatitis b virus is transcribed. Besides, the invention further discloses a construction method and application of the vector. By means of the CAR-T transgene vector and the construction method and application of the vector, secretion of cell factors and an in vitro killing effect of CAR-T cells can be remarkably improved, and the clinical treatment effect is remarkable.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
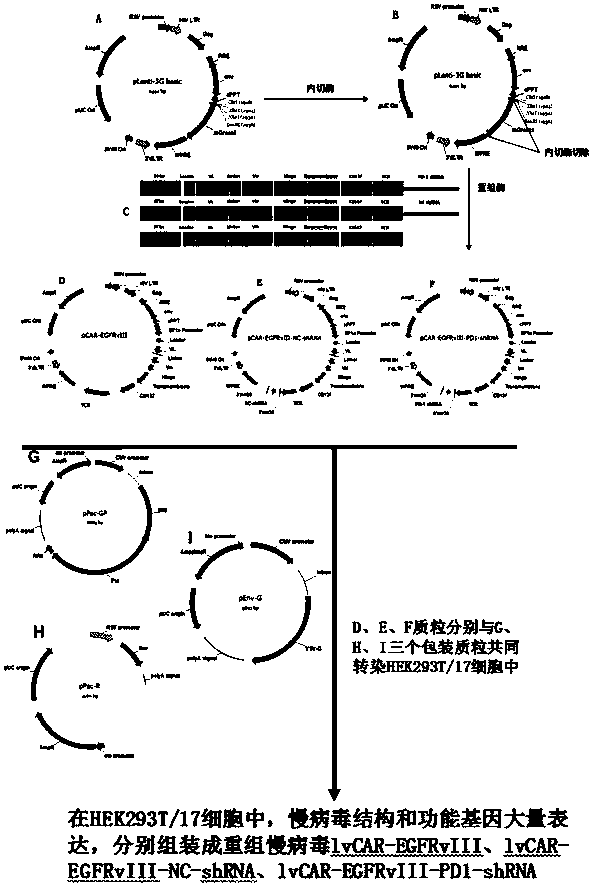
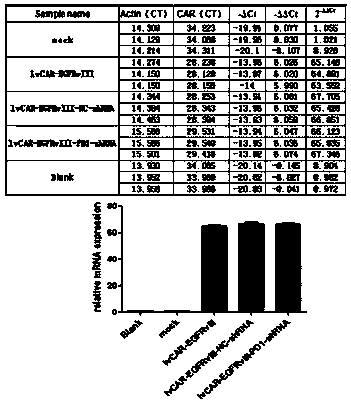
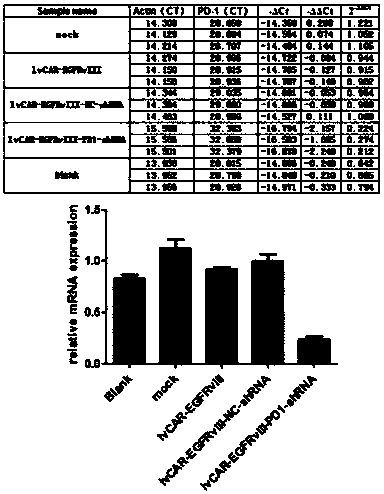
CAR-T (chimeric antigen receptor-T) carrier targeted at EGFRvIII to interfere PD-1 and reduce tumor immune escape and construction method and application
InactiveCN108203718AIncrease lethalityReduce immune escapeMammal material medical ingredientsAntibody ingredientsSingle-Chain AntibodiesImmune escape
The invention discloses a CAR-T carrier targeted at EGFRvIII to interfere PD-1 and reduce tumor immune escape, the CAR-T carrier comprises a human DF1 alpha promoter, PD-1shRNA in 3'UTR, a gene transfer carrier and a second generation CAR or third generation CAR, wherein the human DF1 alpha promoter is used for transcribing CAR genes and shown as SEQ ID NO. 1; and the sequence of the PD-1shRNA isshown as SEQ ID NO. 25; the second generation CAR or third generation CAR is used for integrating recognition, transmitting and starting and comprises an EGFRvIII single-chain antibody light chain VLand an EGFRvIII single-chain antibody heavy chain VH. Besides, the invention discloses a construction method of the carrier and application of the carrier in preparation of drugs for interfering the PD-1 to reduce tumor immune escape. According to the CAR-T carrier targeted at the EGFRvIII to interfere the PD-1 and reduce tumor immune escape and the construction method and the application, the CAR-T technology aiming at the SGFRvIII is used, tumour cell immune escape is inhibited by interfering the expression of the PD-1 in the T lymphocyte, killing to the tumour cells by the T lymphocyte is enhanced, and accordingly, the glioma resistance effect of the CAR-T immunotherapy is improved.
Owner:上海生博生物医药科技有限公司
Anti-her2 chimeric antigen receptor, coding gene, recombinant expression vector and its construction method and application
ActiveCN105949318BImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsMammal material medical ingredientsSingle-Chain AntibodiesTransmembrane domain
The invention discloses an anti-HER2 chimeric antigen receptor, an encoding gene, a recombinant expression vector, a construction method of the recombinant expression vector, and an application. The anti-HER2 chimeric antigen receptor comprises CD8 leader chimeric receptor signal peptide, an HER2 single-chain antibody heavy chain VH, Optimal Linker C, an HER2 single-chain antibody light chain VL, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulated factor and a TCR chimeric receptor T cell activating domain which are connected in series. Moreover, the invention discloses an encoding gene of the anti-HER2 chimeric antigen receptor, a recombinant expression vector, a construction method of the recombinant expression vector, and an application. Secretion of cell factors and an in-vitro killing effect of CAR-T cells are obviously improved, and the CAR-T cells have an outstanding clinical treatment effect.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Anti-BCMA chimeric antigen receptor, coding gene, recombinant expression vector and its construction method and application
ActiveCN105777911BImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsMammal material medical ingredientsSingle-Chain AntibodiesAntigen receptors
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
A car-t therapy vector for pancreatic cancer and malignant mesothelioma based on octs technology and its construction method and application
ActiveCN107299110BExpand the scope of recognitionAvoid batch cultureVirusesPeptide/protein ingredientsSingle-Chain AntibodiesCD8
The present invention discloses a CAR-T therapeutic vector for pancreatic cancer and malignant mesothelioma based on OCTS technology, including lentiviral backbone plasmid, human EF1α promoter (SEQ ID NO.14), OCTS chimeric receptor domain and PDL1 Single-chain antibody; OCTS chimeric receptor domain includes: CD8 leader chimeric receptor signal peptide (SEQ ID NO.15), PDL1 single-chain antibody light chain VL (SEQ ID NO.16), PDL1 single-chain antibody heavy chain VH (SEQ ID NO.17), MESOTHELIN single-chain antibody light chain VL (SEQ ID NO.18), MESOTHELIN single-chain antibody heavy chain VH (SEQ ID NO.19), antibody internal hinge Inner-Linker (SEQ ID NO. 20), Inter-Linker between single-chain antibodies (SEQ ID NO.21), CD8 Hinge chimeric receptor hinge (SEQ ID NO.22), CD8 Transmembrane chimeric receptor transmembrane region (SEQ ID NO.23), TCR chimeric receptor T cell activation domain (SEQ ID NO. 26) and chimeric receptor costimulator region. In addition, the invention also discloses the construction method of the carrier and its application in the preparation of medicines for treating pancreatic cancer and malignant mesothelioma.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Anti-cd138 chimeric antigen receptor, coding gene, recombinant expression vector and its construction method and application
ActiveCN105820254BImprove in vitro killing effectGood clinical effectVirusesAntibody mimetics/scaffoldsSingle-Chain AntibodiesTransmembrane domain
The invention discloses an anti-CD138 chimeric antigen receptor, a coding gene, a recombinant expression vector and a construction method and an application of the recombinant expression vector. The anti-CD138 chimeric antigen receptor comprises a CD8leader chimeric receptor signal peptide, a light chain VH of the CD138 single-chain antibody, an Optimal Linker C, a heavy chain VL of a CD138 single-chain antibody, a CD8Hinge chimeric receptor hinge, a CD8Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulation factor and a TCR chimeric receptor T-cell activation domain, which are serially connected in sequence. In addition, the invention discloses the coding gene of the anti-CD138 chimeric antigen receptor, the recombinant expression vector and the construction method and the application of the recombinant expression vector. According to the anti-CD138 chimeric antigen receptor, the secretion of cell factors and the in vitro killing effect of CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Anti-egfrviii chimeric antigen receptor, coding gene, recombinant expression vector and its construction method and application
ActiveCN105949316BImprove in vitro killing effectGood clinical effectAntibody mimetics/scaffoldsMammal material medical ingredientsSingle-Chain AntibodiesAntigen receptors
The invention discloses an anti-EGFRvIII chimeric antigen receptor, an encoding gene, a recombinant expression vector, a construction method of the recombinant expression vector, and an application. The anti-EGFRvIII chimeric antigen receptor comprises CD8 leader chimeric receptor signal peptide, an EGFRvIII single-chain antibody light chain VL, Optimal Linker C, an EGFRvIII single-chain antibody heavy chain VH, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulated factor and a TCR chimeric receptor T cell activating domain which are connected in series. Moreover, the invention discloses an encoding gene of the anti-EGFRvIII chimeric antigen receptor, a recombinant expression vector, a construction method of the recombinant expression vector, and an application. Secretion of cell factors and an in-vitro killing effect of CAR-T cells are obviously improved, and the CAR-T cells have an outstanding clinical treatment effect.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Anti-cd20 chimeric antigen receptor, coding gene, recombinant expression vector and its construction method and application
ActiveCN105949317BImprove in vitro killing effectGood clinical effectMammal material medical ingredientsImmunoglobulinsSequence signalSingle-Chain Antibodies
The invention discloses an anti-CD20 chimeric antigen receptor, an encoding gene, a recombinant expression vector, a construction method of the recombinant expression vector and an application. The anti-CD20 chimeric antigen receptor comprises CD8 leader chimeric receptor signal peptide, CD20 VL, Optimal Linker C, a CD20 single-chain antibody heavy chain VH, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulated factor and a TCR chimeric receptor T cell activating domain which are connected in series. Moreover, the invention discloses an encoding gene of the anti-CD20 chimeric antigen receptor, a recombinant expression vector, a construction method of the recombinant expression vector, and an application. Secretion of cell factors and an in-vitro killing effect of CAR-T cells are obviously improved, and the CAR-T cells have an outstanding clinical treatment effect.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Car-t therapy carrier for malignant glioma based on octs technology and its construction method and application
ActiveCN107267555BExpand the scope of recognitionAvoid batch cultureVirusesPeptide/protein ingredientsSingle-Chain AntibodiesReceptor
The present invention discloses a malignant glioma CAR-T therapeutic vector based on OCTS technology, including lentiviral backbone plasmid, human EF1α promoter (SEQ ID NO.14), OCTS chimeric receptor domain and PDL1 single-chain antibody ; OCTS chimeric receptor domain includes: CD8 leader chimeric receptor signal peptide (SEQ ID NO.15), PDL1 single-chain antibody light chain VL (SEQ ID NO.16), PDL1 single-chain antibody heavy chain VH (SEQ ID NO.16) ID NO.17), EGFRvIII single-chain antibody light chain VL (SEQ ID NO.18), EGFRvIII single-chain antibody heavy chain VH (SEQ ID NO.19), antibody internal hinge Inner-Linker (SEQ ID NO.20), Single-chain antibody Inter-Linker (SEQ ID NO.21), CD8 Hinge chimeric receptor hinge (SEQ ID NO.22), CD8 Transmembrane chimeric receptor transmembrane region (SEQ ID NO.23), TCR chimeric receptor Synthetic receptor T cell activation domain (SEQ ID NO.26) and chimeric receptor co-stimulatory factor region. In addition, the invention also discloses the construction method of the carrier and its application in the preparation of medicine for treating malignant glioma.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
A myeloid leukemia car-t therapeutic vector based on octs technology and its construction method and application
ActiveCN107177632BExpand the scope of recognitionAvoid batch cultureMammal material medical ingredientsNGF-receptor/TNF-receptor superfamilyMyeloid leukemiaSingle-Chain Antibodies
The invention discloses a myeloid leukemia CAR-T treatment vector based on OCTS technology, including lentiviral backbone plasmid, human EF1α promoter (SEQ ID NO. 14), OCTS chimeric receptor domain and PDL1 single chain antibody; The OCTS chimeric receptor domain includes: CD8 leader chimeric receptor signal peptide (SEQ ID NO.15), CD33 single chain antibody light chain VL (SEQ ID NO.16), CD33 single chain antibody heavy chain VH (SEQ ID NO.17), CD123 single-chain antibody light chain VL (SEQ ID NO.18), CD123 single-chain antibody heavy chain VH (SEQ ID NO.19), intra-antibody hinge Inner‑Linker (SEQ ID NO.20), single-chain antibody Inter-Linker (SEQ ID NO.21), CD8 Hinge chimeric receptor hinge (SEQ ID NO.22), CD8 Transmembrane chimeric receptor transmembrane region (SEQ ID NO.23), TCR chimeric Receptor T cell activation domain (SEQ ID NO. 26) and chimeric receptor costimulator region. In addition, the present invention also discloses the construction method of the vector and its application in preparing drugs for treating myeloid leukemia.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
A replication-defective recombinant lentiviral car-t transgene vector targeting CD22 and its construction method and application
ActiveCN105950662BPromote secretionImprove in vitro killing effectMammal material medical ingredientsNucleic acid vectorAmpicillinFluorescence
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com