A car-t therapy vector for pancreatic cancer and malignant mesothelioma based on octs technology and its construction method and application
A technology for malignant mesothelioma and pancreatic cancer, applied in the field of medical biology, can solve problems that have not been overcome, and achieve the effects of improving curative effect, inhibiting immune escape, and reliable protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0052] Below in conjunction with specific embodiment further elaborates this invention. It should be understood that the specific embodiments described herein are presented by way of example and not as limitations of the invention. The principal characteristics of this invention can be employed in various embodiments without departing from the scope of the invention. Material
[0053] 1. Lentiviral backbone plasmid pLenti-3G basic, lentiviral packaging plasmids pPac-GP, pPac-R and membrane protein plasmid pEnv-G, HEK293T / 17 cells, homologous recombination enzyme, Oligo Annealing Buffer, mycoplasma detection kit, internal Toxin Detection Kit, PDL1 + K562, MESOTHELIN + K562, PDL1 + MESOTHELIN + K562 and K562 cells were purchased from Shiao (Shanghai) Biomedical Technology Co., Ltd.; the specific preparation method of the lentiviral backbone plasmid pLenti-3G basic has been published in the invention titled "A CAR-T transgene based on replication-defective recombinant le...
Embodiment 1
[0094] Example 1 Construction of OCTS-CAR-T cells
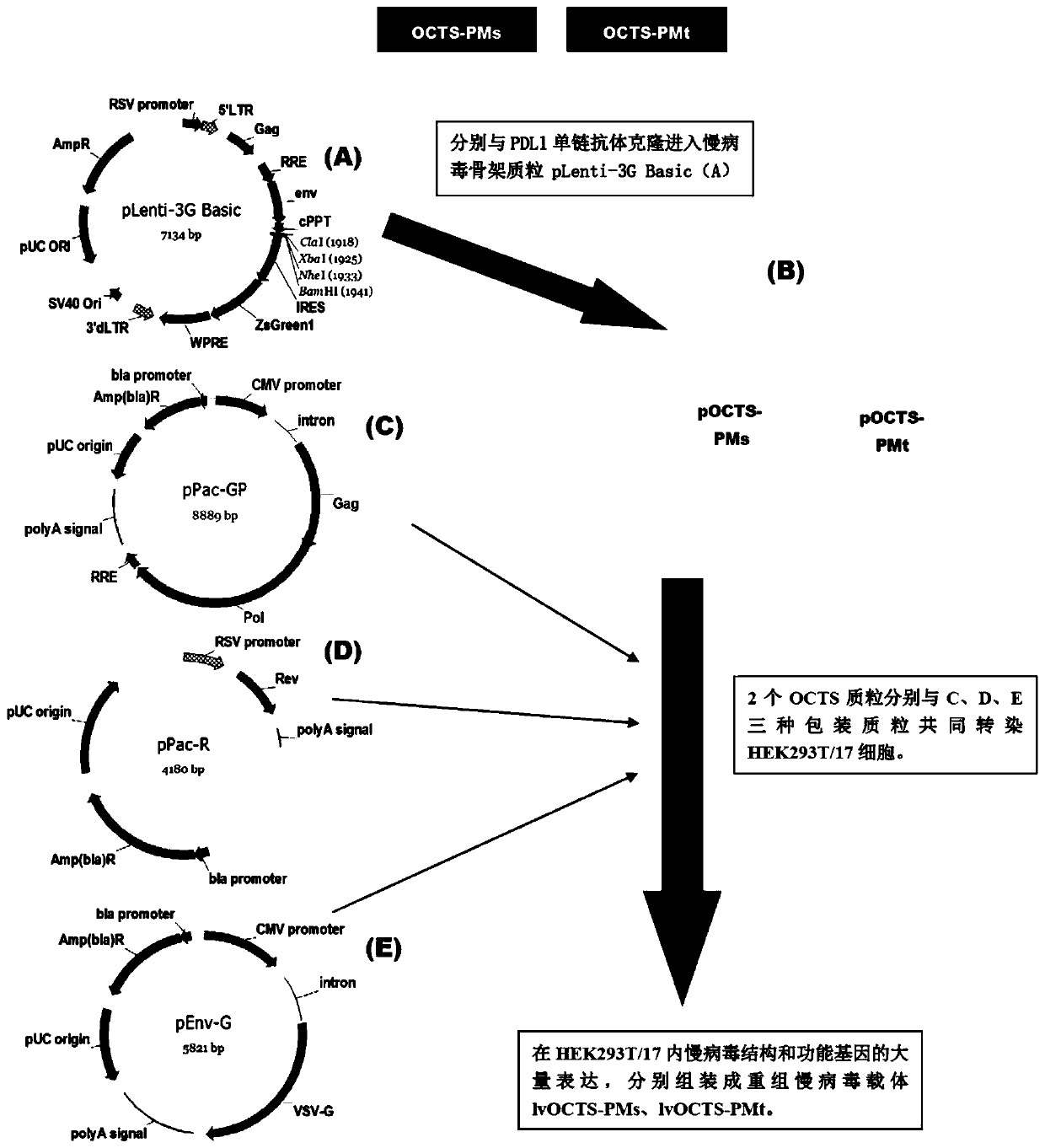
[0095] 1. Construction, purification and detection methods of recombinant lentiviral vectors lvOCTS-PMs and lvOCTS-PMt.
[0096] see image 3 , the construction method of the recombinant lentiviral vector of the present invention is as follows:
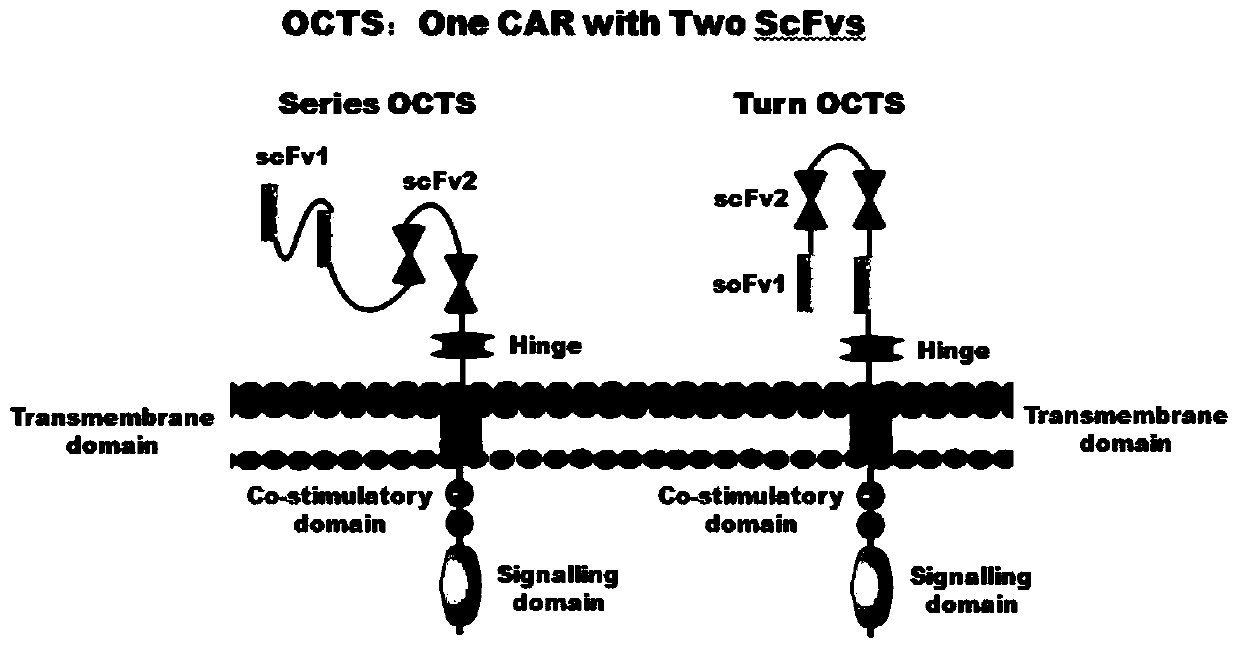
[0097] 1. Human EF1α promoter (SEQ ID NO.14), OCTS structure [OCTS-PMs, OCTS-PMt] (CD8leader chimeric receptor signal peptide (SEQ ID NO.15), PDL1 single-chain antibody light chain VL ( SEQ ID NO.16), PDL1 single-chain antibody heavy chain VH (SEQ ID NO.17), MESOTHELIN single-chain antibody light chain VL (SEQ ID NO.18), MESOTHELIN single-chain antibody heavy chain VH (SEQ ID NO.19 ), the hinge Inner-Linker (SEQ ID NO.20) in the antibody, the hinge Inter-Linker (SEQ ID NO.21) between single-chain antibodies, the CD8Hinge chimeric receptor hinge (SEQ ID NO.22), the CD8Transmembrane chimeric receptor body transmembrane region (SEQ ID NO.23), CD28 chimeric receptor costimulator (SEQ ...
Embodiment 2
[0204] OCTS-CAR-T cell pathogen detection and expression detection.
[0205] 1. Endotoxin detection;
[0206] (1), endotoxin working standard is 15EU / branch;
[0207] (2), Limulus reagent sensitivity λ=0.25EU / ml, 0.5ml / tube
[0208] (3) Dilution of endotoxin standard substance: Take one endotoxin standard substance, dilute it with BET water in proportion to dissolve into 4λ and 2λ respectively, seal with parafilm, shake and dissolve for 15min; each step of dilution should be mixed in the vortex Mix on the mixer for 30s;
[0209] (4) Adding samples: Take several LAL reagents, add 0.5 ml of BET water to each tube to dissolve, and distribute to several endotoxin-free test tubes, each tube has 0.1 ml. Two of them are negative control tubes, add 0.1ml of BET water;
[0210] Two are positive control tubes, add 0.1ml of endotoxin working standard solution with 2λ concentration;
[0211] 2 tubes are sample positive control tubes, add 0.1ml sample solution containing 2λ endotoxin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com