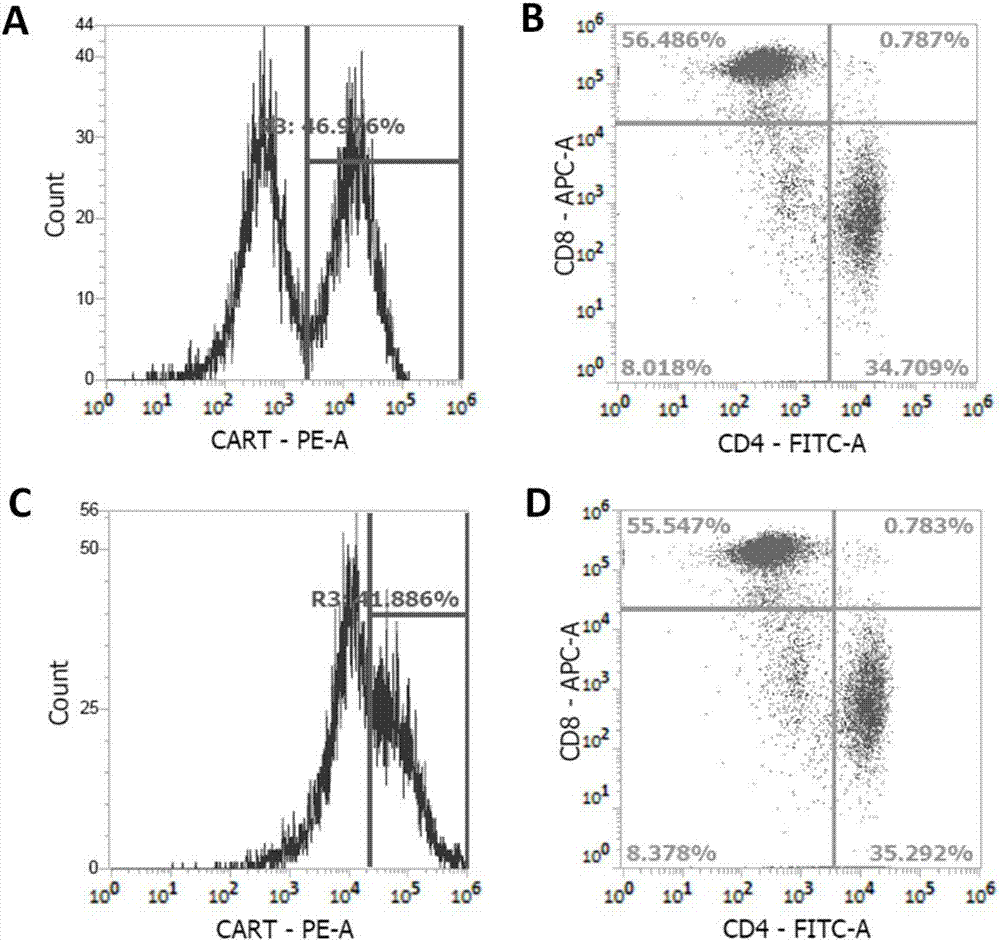
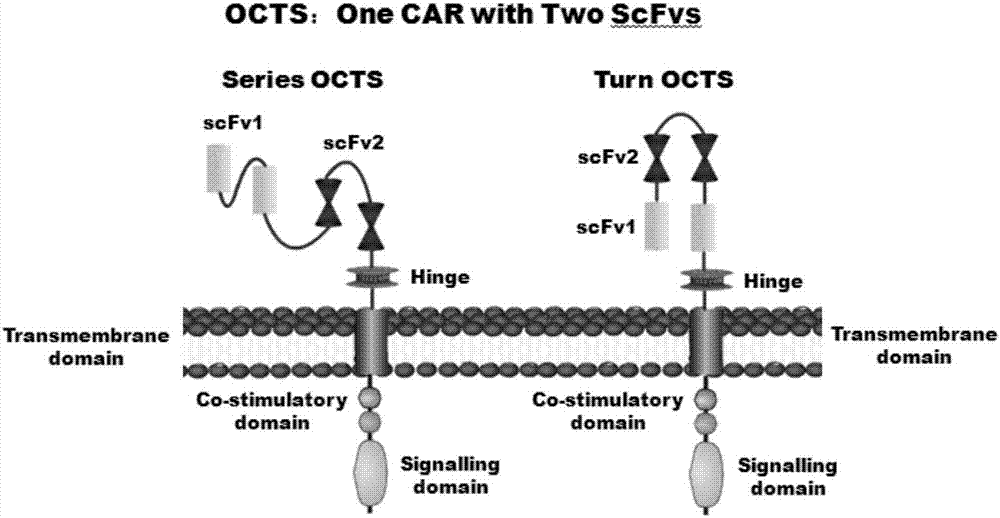
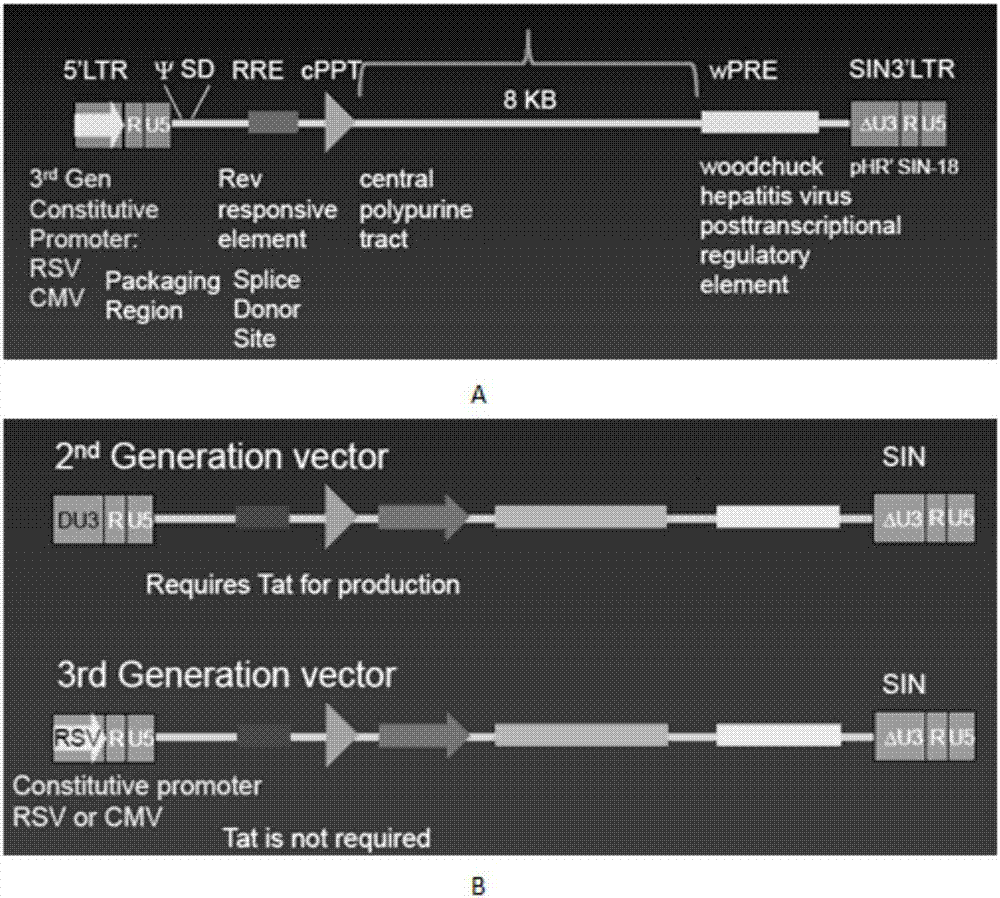
OCTS technology-based prostatic cancer CAR-T therapy vector and construction method and application thereof
A prostate cancer and carrier technology, applied in the field of medical biology, can solve problems that have not yet been overcome, and achieve the effects of saving economic expenses, improving quality of life, and high transduction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1 Construction of OCTS-CAR-T cells
[0095] 1. Construction, purification and detection methods of recombinant lentiviral vectors lvOCTS-PDL1PSMAs and lvOCTS-PDL1PSMAt.
[0096] see image 3 , the construction method of the recombinant lentiviral vector of the present invention is as follows:
[0097] 1. Human EF1α promoter (SEQ ID NO.14), OCTS structure [OCTS-PDL1PSMAs, OCTS-PDL1PSMAt] (CD8 leader chimeric receptor signal peptide (SEQ ID NO.15), PSMA single-chain antibody light chain VL (SEQ ID NO.16), PSMA single-chain antibody heavy chain VH (SEQ ID NO.17), PDL1 single-chain antibody light chain VL (SEQ ID NO.18), PDL1 single-chain antibody heavy chain VH (SEQ ID NO.19 ), the hinge Inner-Linker (SEQ ID NO.20) in the antibody, the hinge Inter-Linker (SEQ ID NO.21) between single-chain antibodies, the CD8Hinge chimeric receptor hinge (SEQ ID NO.22), the CD8Transmembrane chimeric receptor body transmembrane region (SEQ ID NO.23), CD28 chimeric receptor costim...
Embodiment 2
[0206] OCTS-CAR-T cell pathogen detection and expression detection.
[0207] 1. Endotoxin detection;
[0208] (1), endotoxin working standard is 15EU / branch;
[0209] (2), Limulus reagent sensitivity λ=0.25EU / ml, 0.5ml / tube
[0210] (3) Dilution of endotoxin standard substance: Take one endotoxin standard substance, dilute it with BET water in proportion to dissolve into 4λ and 2λ respectively, seal with parafilm, shake and dissolve for 15min; each step of dilution should be mixed in the vortex Mix on the mixer for 30s;
[0211] (4) Adding samples: Take several LAL reagents, add 0.5 ml of BET water to each tube to dissolve, and distribute to several endotoxin-free test tubes, each tube has 0.1 ml. Two of them are negative control tubes, add 0.1ml of BET water;
[0212] Two are positive control tubes, add 0.1ml of endotoxin working standard solution with 2λ concentration;
[0213] 2 tubes are sample positive control tubes, add 0.1ml sample solution containing 2λ endotoxin ...
Embodiment 3
[0243] Example 3 Functional testing of OCTS-CAR-T cells.
[0244] 1. Evaluation of target cell killing effect.
[0245] (1) Culture target cells separately [PSMA + K562, PDL1 + K562, PDL1 + PSMA + K562, K562 cells] and effector cells [OCTS-CAR-T cells];
[0246] (2) Collect target cells 4x10 5 cells and OCTS-CAR-T cells 2.8x10 6 cells, 800g, centrifuge for 6min, discard the supernatant;
[0247] (3) Resuspend the target cells and effector cells in 1ml D-PBS(-) solution, centrifuge at 800g for 6min, discard the supernatant;
[0248] (4) Repeat step 3 once;
[0249] (5) Resuspend effector cells with 700ul medium (AIM-V medium + 1-10% FBS), and resuspend target cells with 2ml medium (AIM-V medium + 1-10% FBS);
[0250] (6) Experimental wells with effect-to-target ratios of 1:1, 5:1, and 10:1 were set, and the grouping of effector cells co-incubated with single-target cells and double-target cells was shown in Table 6, and a control group ( K562 cells), 3 replicate wells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com