Application of progestational hormone in treatment of cytokine release syndrome
A technology of cytokines and syndromes, applied in the field of treatment of immune diseases and infectious diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
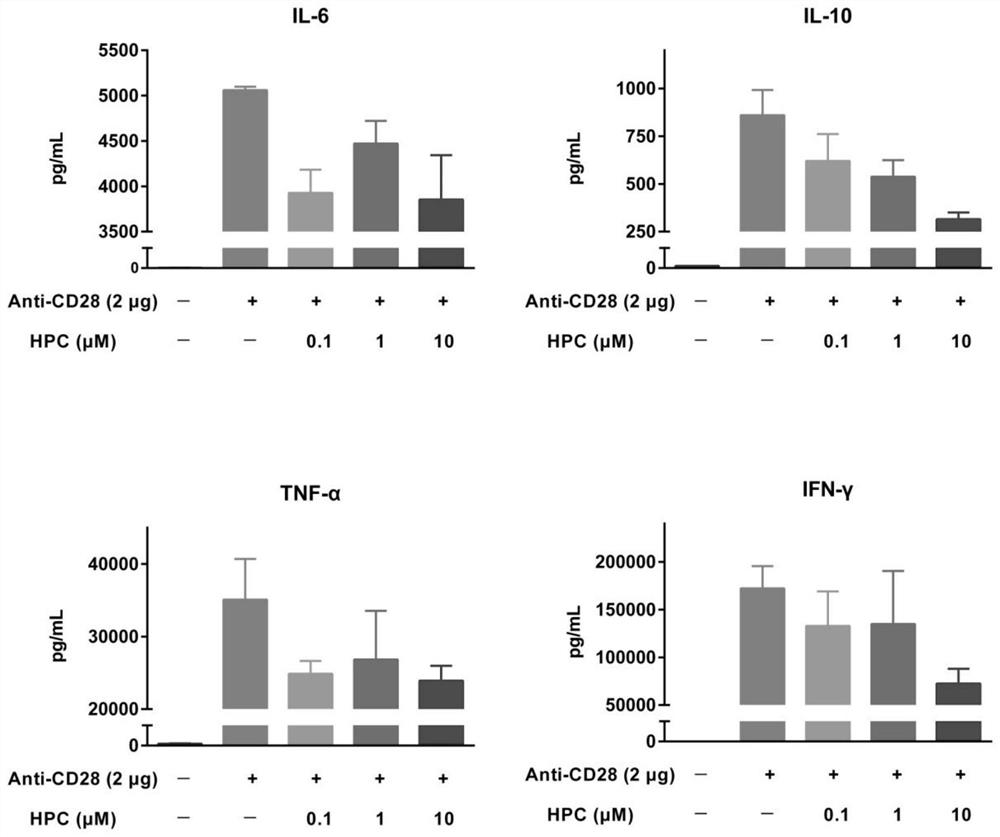
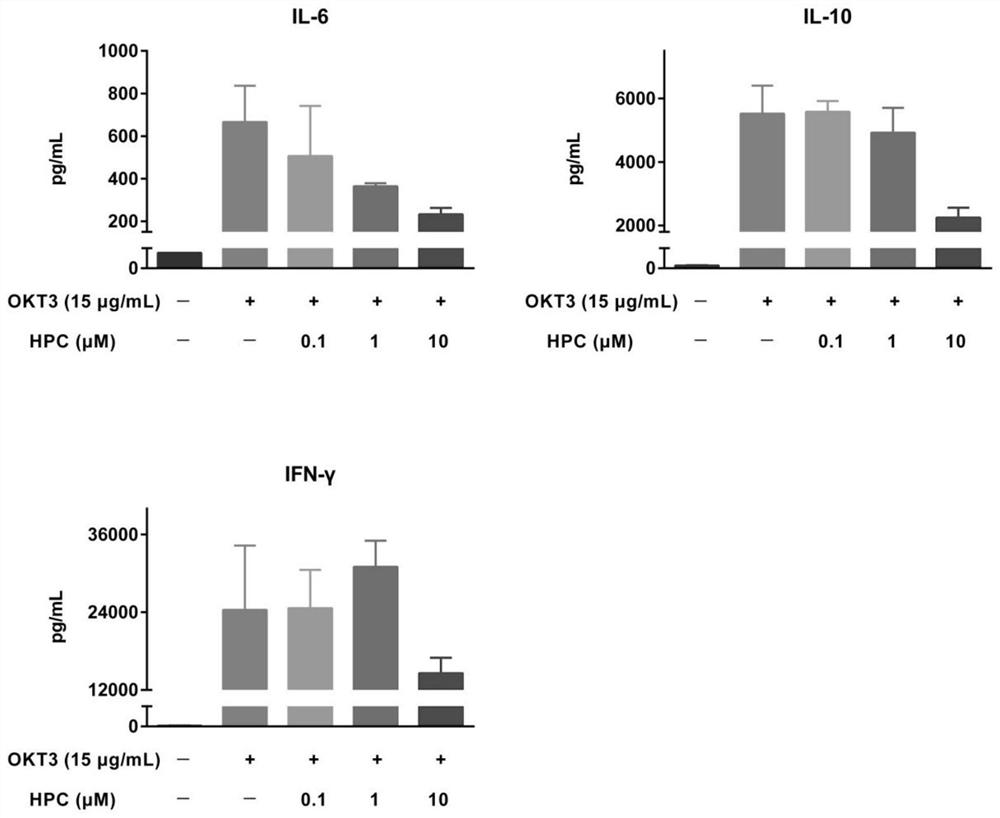
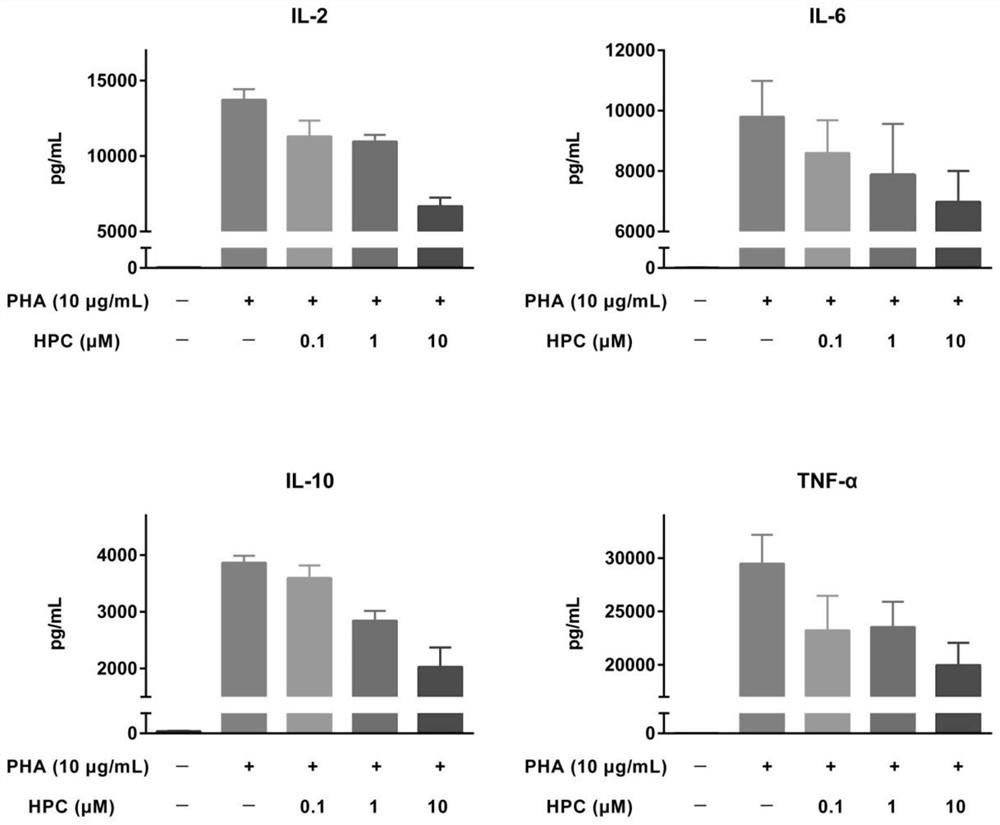
[0025] Example 1 Hydroxyprogesterone Caproate (HPC) Inhibits Cytokine Release in Human Peripheral Blood Mononuclear Cells (PBMCs)
[0026] 1) T cell inflammation suppression experimental method using anti-CD28 superagonist
[0027] first day
[0028] The anti-CD28 superagonist (clone ANC28.1 / 5D10, 2 μg / well) dissolved in PBS 1x was inoculated in a high-binding microtiter plate, incubated overnight in a biological safety cabinet with the cover open, fixed and air-dried. Frozen PBMCs were thawed, mixed and diluted to an appropriate density, inoculated into a U-shaped bottom polypropylene 96-well plate (1.2x105 cells per well), and 228 μL of medium (RPMI 1640, 10% heat-inactivated embryo bovine serum, 1% penicillin / streptomycin, 2mM L-glutamine). Cells were incubated at 37°C, 5% CO2 for 1 hour before adding HPC. HPC was dissolved in 50% ethanol & PBS and further diluted with 20X cell culture medium. Add HPC to PBMCs, repeat three groups in parallel, each group 12 μL (1X), 37 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com