Universal chimeric antigen receptor-T (CAR-T) cell as well as preparation method and application thereof
A universal, cellular technology, applied in the field of preparation, universal CAR-T cells, to save time and cost, shorten preparation time, and reduce preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of universal CAR-T cells (svCAR-T)
[0039] (1) Synthesizing the anti-GCN single-chain antibody region, the sequence of which is shown in SEQ ID NO:1;
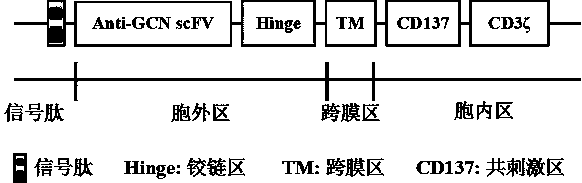
[0040](2) Obtaining of the Hinge-TM-CD137-CD3ζ gene sequence: design and synthesize the Hinge-TM-CD137-CD3ζ expression cassette of the chimeric antigen receptor according to the CD8α, CD137 and CD3ζ gene sequences, including the signal peptide (carrying the gene sequence such as SEQ IDNO: 6), hinge region (carrying gene sequence as shown in SEQ ID NO: 5), CD3ζ region (carrying gene sequence as shown in SEQ ID NO: 2), transmembrane region (carrying gene sequence as shown in SEQ ID NO: 4 shown), the co-stimulatory region (carrying the gene sequence shown in SEQ ID NO:3) and the anti-GCN single-chain antibody region (carrying the sequence of SEQ ID NO:1) were gene synthesized, and the obtained plasmid was named pCD-HTCC.
[0041] (3) Hinge-TM-CD137-CD3ζ was connected with vector pCDH-CMV-MCS to obtain vector p...
Embodiment 2
[0047] Preparation of GCN-Autoantibody Affinity Peptide Fusion Polypeptide (GCN-sP) Leader
[0048] 1) The polypeptide GCN is derived from the 14 amino acid peptides of the yeast transcription factor GCN4, which can bind with high affinity to the front end of svCAR anti-GCNscFV, and can guide and bridge the combination of svCAR-T and target autoreactive B cells. Its sequence is shown in SEQ ID NO: 7.
[0049] 2) The linker is used to connect the polypeptide GCN and the autoantibody affinity peptide, and its sequence is shown in SEQ ID NO:8.
[0050] 3) Candidate autoantibody affinity peptides are identified autoimmune nephropathy-related autoantigen epitope peptides or mimetic peptides:
[0051] IMN affinity peptide Pp: the sequence is shown in SEQ ID NO:19;
[0052] IgAN affinity peptide Pa: the sequence is shown in SEQ ID NO:20;
[0053] LN affinity peptide Pd: the sequence is shown in SEQ ID NO:21;
[0054] MPO-AAV affinity peptide Pma: the sequence is shown in SEQ ID N...
Embodiment 3
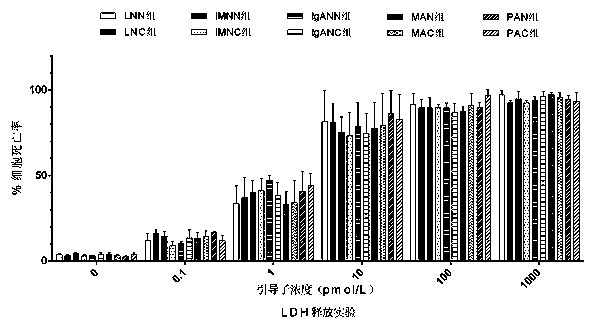
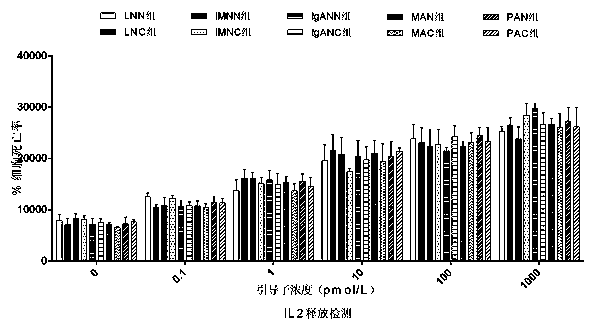
[0069] Affinity Test of GCN-sP Series Peptides and Autoantibodies by Enzyme-Linked Immunosorbent Assay
[0070] 1) Preparation of GCN-sP and control peptides: The affinity of autoantibodies between 10 kinds of GCN-sP synthesized in Example 2 and 5 kinds of control peptides (Pp, Pa, Pd, Pma, Ppa) was detected.
[0071] 2) Negative and positive serum preparation:
[0072] 30 cases of serum from LN patients confirmed to contain anti-dsDNA antibodies by clinical tests;
[0073] ●Thirty cases of serum from IMN patients confirmed to contain anti-PLA2R antibody by clinical tests;
[0074] 30 cases of sera from IgAN patients with positive Gd-IgA1 confirmed by human Gd-IgA1 detection kit (27600, Japan IBL);
[0075] ●Thirty cases of serum from MPO-AAV patients confirmed to contain MPO-ANCA antibody by clinical tests;
[0076] ●Thirty cases of serum from PR3-AAV patients confirmed to contain PR3-ANCA antibody by clinical tests;
[0077] ·Negative control serum is normal human serum....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com