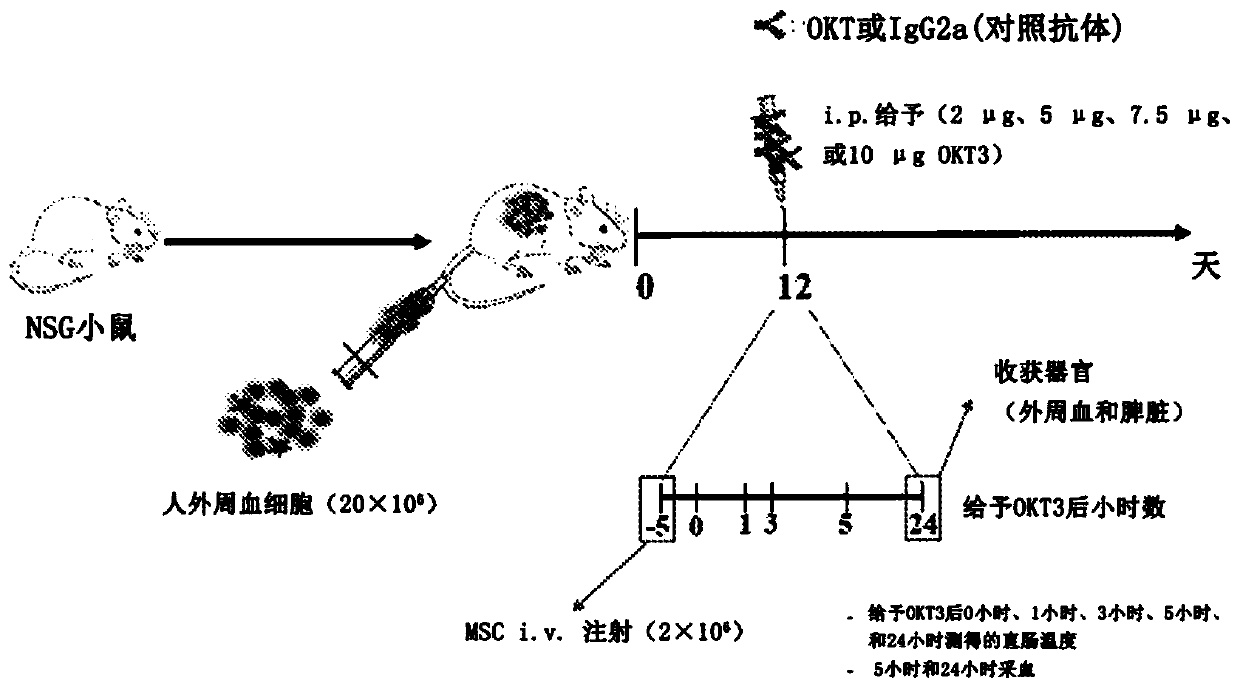
Method for treating side effect of chimeric antigen receptor (CAR) T cell therapy
A cell therapy, side effect technology, applied in receptors/cell surface antigens/cell surface determinants, antibodies, animal cells, etc., can solve the problems of subject death and incomplete success
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1. CAR and retroviral vectors
[0128] All CARs in these examples comprised scFv derived from the J591 hybridoma specific for prostate-specific membrane antigen (PSMA) as described (Gong, M.C. et al. Neoplasia 1, 123-127 (1999)). sex. To facilitate detection of transduced cells, all constructs contained the encephalomyocarditis virus internal ribosome entry site (EMCV-IRES) and the eGFP gene inserted into the SFG vector. In Pz1, J591 scFv(P) is coupled to the intracellular domain of human TCRζ(z) through the human CD8α hinge and transmembrane sequence (Gong et al.). P28 comprises a fusion of J591 scFv (P) to human CD28 (28) as described (Krause, A. et al. J. Exp. Med. 188, 619-626 (1998) and Krause, A. et al. Mol. Ther. 1 , S260, 713 (2000)). To construct P28z, primers 5'-GGCGGCCGCAATTGAAGTTATGTATC-3' (SEQ ID NO: 1) and 5'-TGCGCTCCTGCTGAACTTCACTCTGGAGCGATAGGCTGCTAAGTCGCG-3 (SEQ ID NO: 2) were used to amplify nucleotides 336-660 of CD28. The intracellular dom...
Embodiment 2
[0129] Example 2. Culture and Retroviral Transduction of Primary Human T Cells
[0130] Peripheral blood mononuclear cells from healthy donors were established in RPMI+10% (vol / vol) human serum, activated with lectin (2 μg / ml) for two days, and transferred to cells pre-coated with Retronectin (15 μg / ml; Non-tissue culture plates (FALCON, Becton Dickinson, Franklin, Lakes, N.J.) from Takara Biomedicals, Shiga, Japan). Gibbon leukemia virus envelope pseudotyped retroviral particles comprising the CAR construct were generated as described (Gallardo, H.F. et al. Blood 90, 952-957 (1997) and Rivière, I. et al. Mol. Biotechnol. )).
[0131] Three days after transduction of mitogen-activated PBLs, gene transfer efficiencies ranged from 20% to 70%. CD4 + and CD8 + T cell subsets were transduced with similar efficiency. Expression of fusion receptors comprising the zeta chain was also analyzed by Western blot, demonstrating the formation of homodimers and little, if any, heterodim...
Embodiment 3
[0132] Example 3. CD19-specific scFv
[0133] To construct CD19-specific scFv, the heavy (VH) and light (VL) chain variable regions were cloned from the hybridoma cell line SJ25C1 using degenerate primers as described (Orlandi et al. Proc Natl Acad Sci USA 86, 3833-3837 (1989)). Combine these coding regions with coding (Gly 3 Ser) 4 The DNA fragments of the spacer are fused. A co-stimulatory signaling element (including transmembrane and extracellular portion) (SEQ ID NO:7) from human CD28 was ligated to the 3' end of the resulting scFv, and the cytoplasmic domain of human ζ (SEQ ID NO:8 ) to the 3' end of the CD28 portion to form the fusion gene 19-28z.
[0134]The 19-28z fusion was tested for its ability to reduce tumor growth and enhance survival in mice injected with NALM6T cells. NALM6 cells express CD19, MHC I, and MHC II, but not B7.1 or B7.2. Most (approximately 80%) of untreated SCID-Beige mice developed hindlimb paralysis 4-5 weeks after tumor cell injection, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com