Method for analyzing amine substances in dansyl chloride derived-plasma based on liquid chromatography mass spectrometry
A technology of dansyl chloride and analytical method, applied in the fields of analytical chemistry and medicine, can solve the problems of small coverage and less amine substances, and achieve the effects of less reagent consumption, high repeatability and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
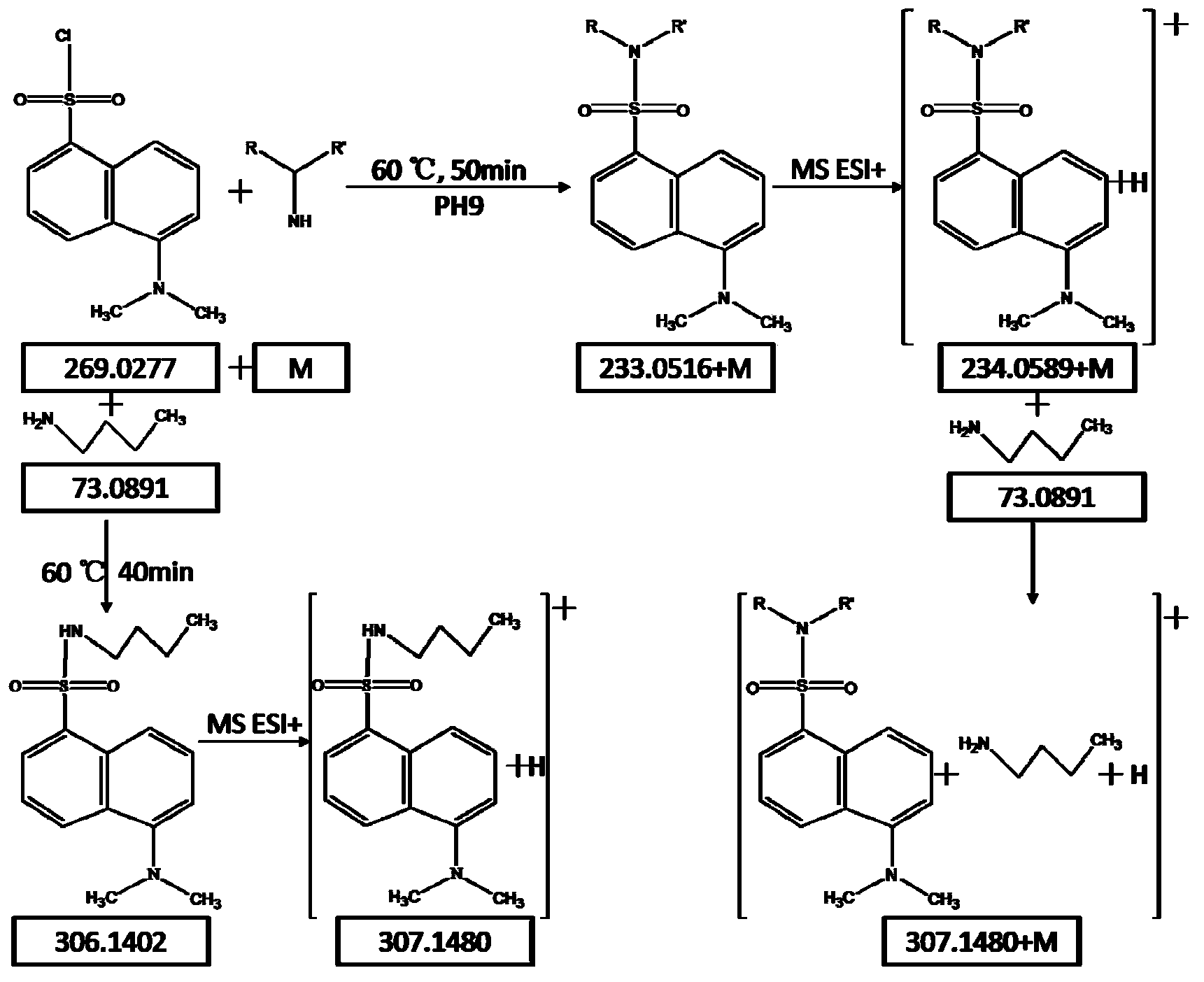
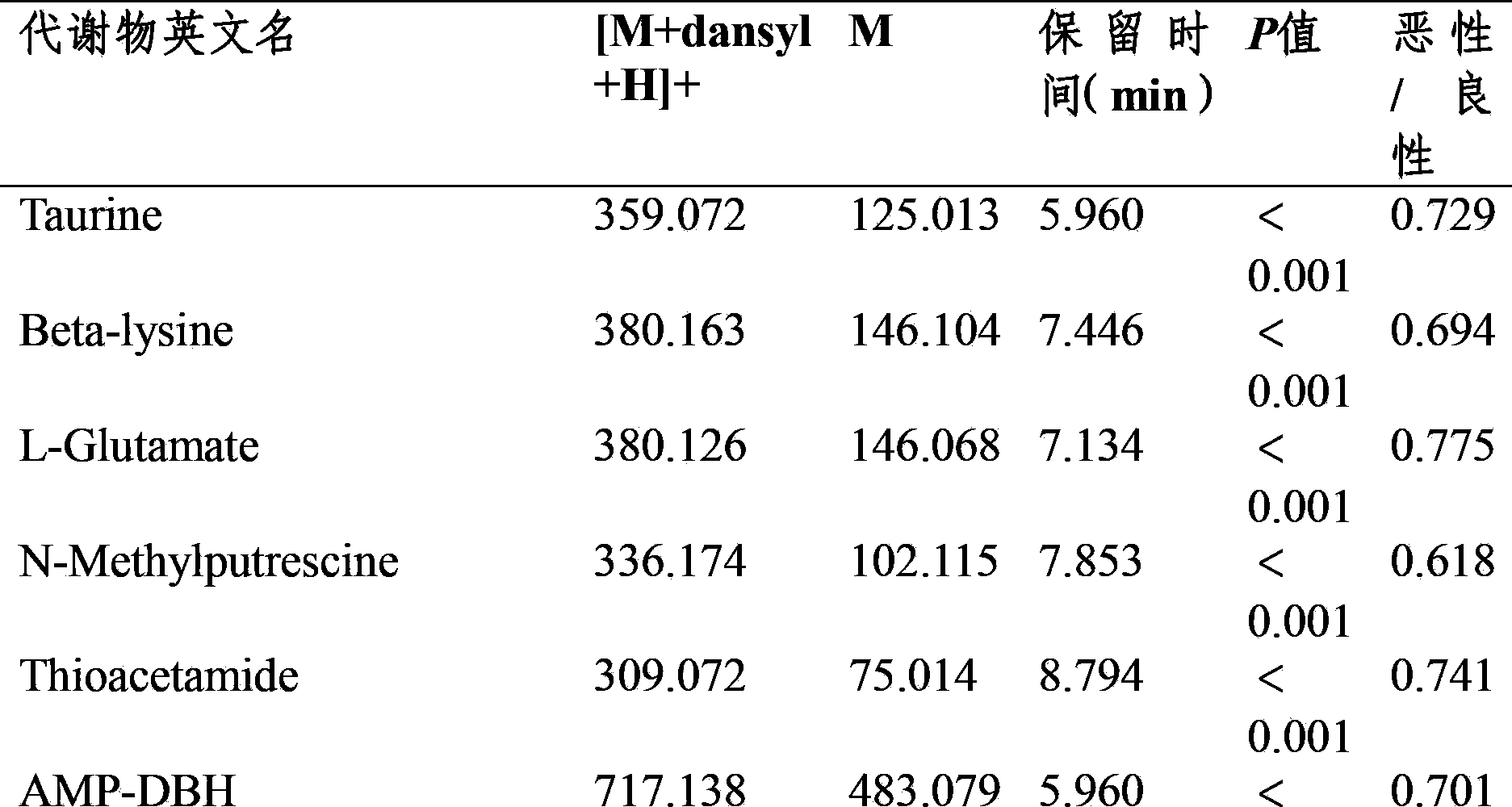
[0022] Thaw the plasma in the -80°C refrigerator to room temperature, quantitatively pipette 100 μL of plasma to a centrifuge tube and add isotope-containing internal standards Glycine-d5, DL-Alanine-3,3,3-d3, L-Leucine-5,5, 400 μL of acetonitrile solution of 5-d3, Thymine-d3, Phenylalanine-d5 and Tryptophan-d5 (400 ng / mL concentration). 17 kinds of amine substances were formulated as a mixed standard of 50ng / mL, and 6 kinds of isotopic internal standards with a concentration of 400ng / mL were added as above. After the two mixtures were vortexed for 1 min respectively, the samples were centrifuged at 15000 rpm / min at 4°C for 10 min. Pipette a volume of 450 μL of the supernatant to a centrifuge tube and lyophilize on a lyophilizer. Add 40 μL of 5 mg / mL dansyl chloride acetonitrile solution and 40 μL of 0.15 mol / L Na to the lyophilized plasma extract 2 CO 3 / NaHCO 3 Aqueous solution (PH=9.4), vortex for 1min, seal the centrifuge tube and then react in a water bath at 60°C for...
Embodiment 2
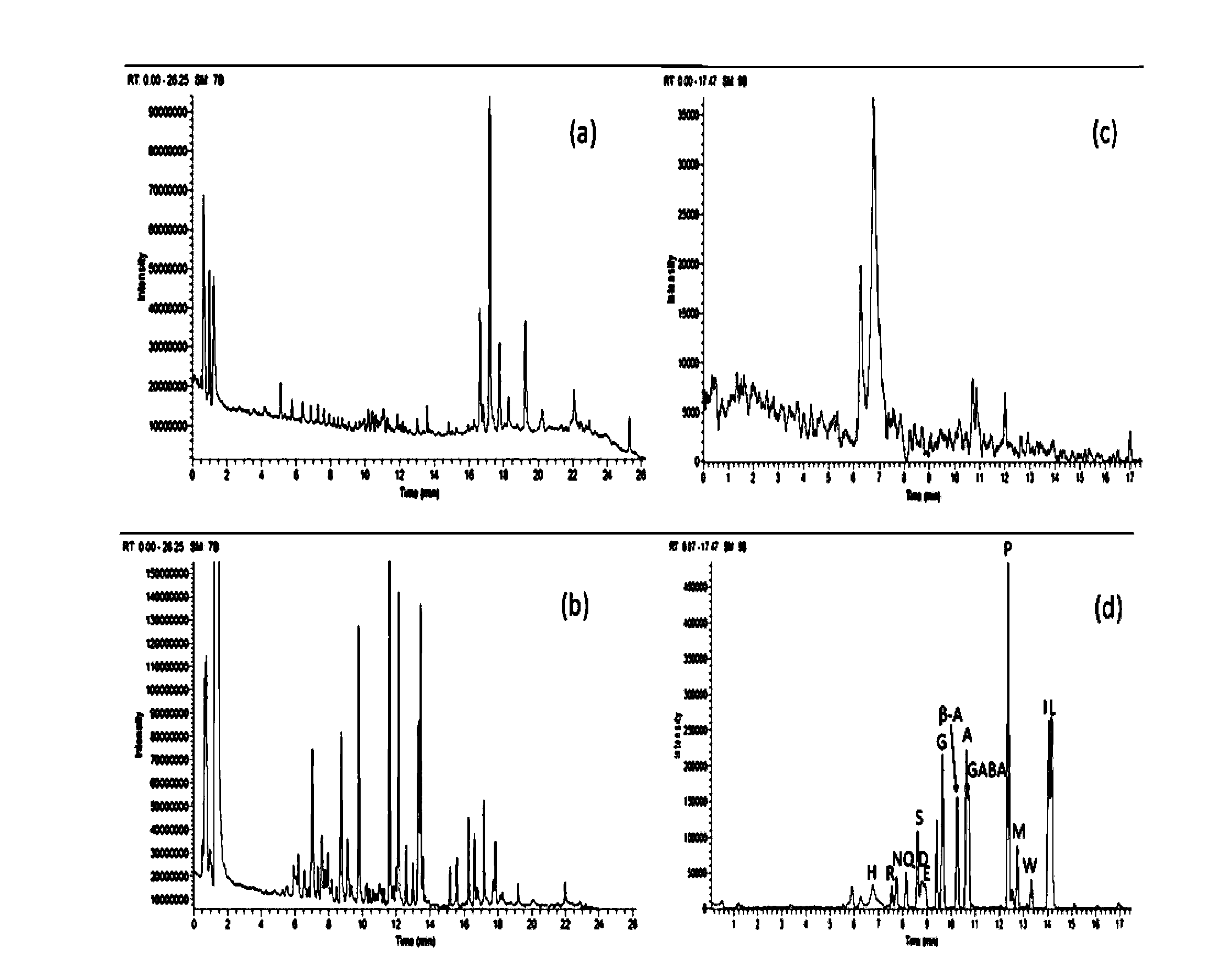
[0027] Before plasma collection, pathological diagnosis was performed by multi-point puncture to ensure the accuracy of sampling. The patients fasted for 12 hours and collected in the morning on an empty stomach. The samples included the plasma of patients with benign prostatic hyperplasia and malignant prostate cancer (ie, patients with benign prostatic hyperplasia and malignant prostate cancer whose PSA could not be distinguished) with a prostate-specific antigen (PSA) concentration of 4-10 ng / mL. After collection, they were quickly stored in a -80°C refrigerator.
[0028] Before analysis, thaw the plasma to room temperature, quantitatively pipette 100 μL of plasma to a centrifuge tube and add internal standard Glycine-d5, DL-Alanine-3,3,3-d3, L-Leucine-5,5,5-d3, Thymine-d3, Phenylalanine-d5 and Tryptophan-d5 (concentration: 400ng / mL) acetonitrile solution 400μL. After vortexing for 1 min, the sample was centrifuged at 15,000 rpm / min at 4°C for 10 min. Pipette a volume of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com