Detection of drug concentration of osimertinib in human plasma and/or cerebrospinal fluid by combining UPLC-MS/MS
A technology for human plasma and cerebrospinal fluid, which is applied in measurement devices, instruments, scientific instruments, etc., can solve the problem of not measuring the concentration of osimertinib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
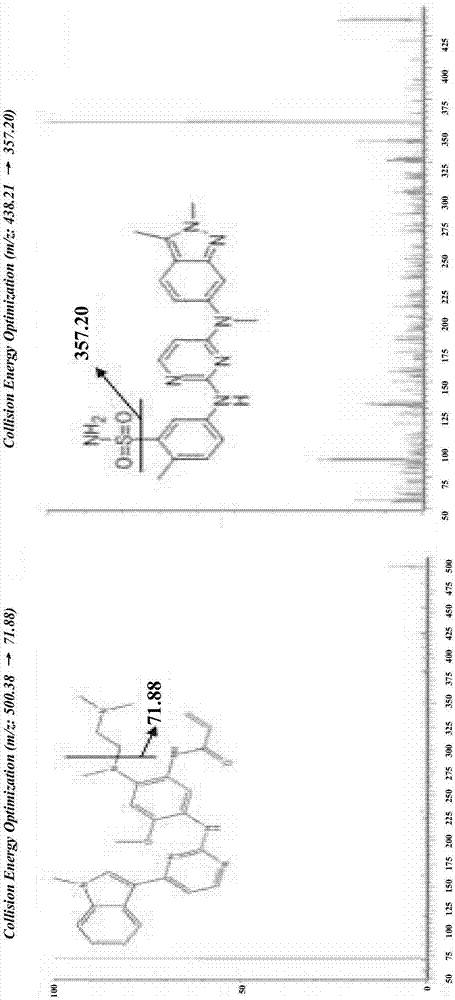
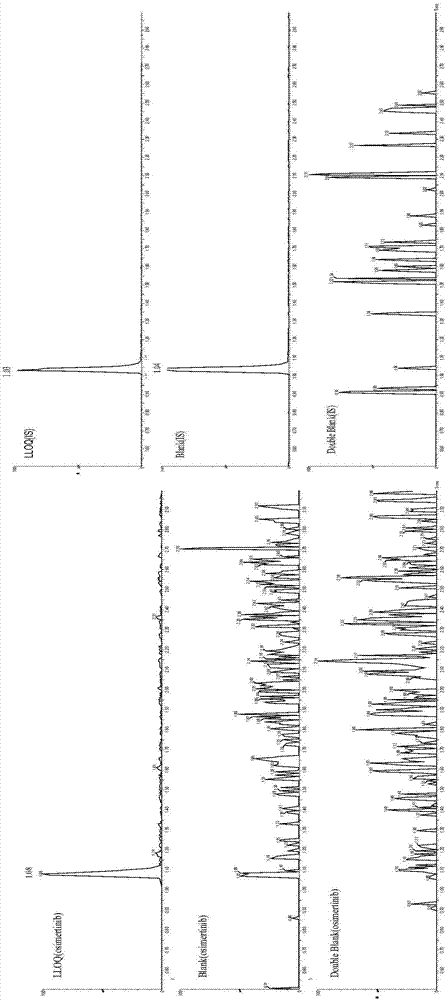
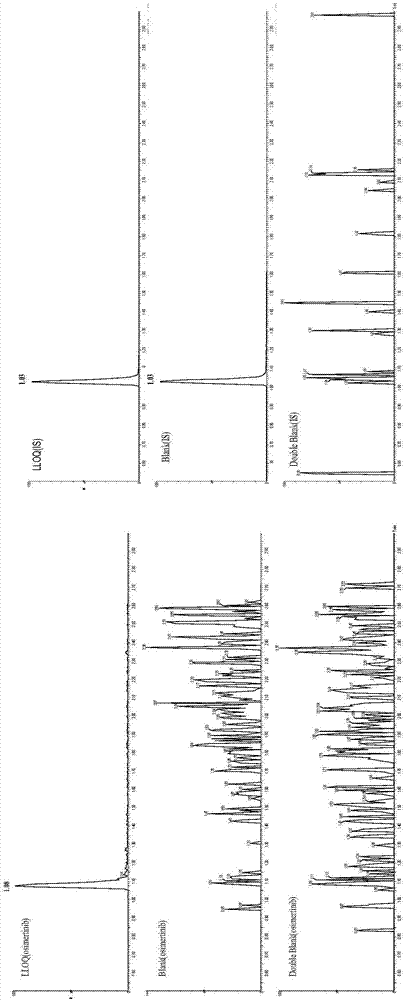
[0042] In the method of the present invention, the following preferred conditions can be considered. The method includes the treatment of plasma and cerebrospinal fluid samples with acetonitrile protein precipitation. The liquid phase conditions are ACQUITY UPLC BEH C18 (50mm×2.1mm, 1.7μm), and the mobile phase is A: 0.1% formic acid 10mM ammonium formate water; B: 0.5% Formic acid acetonitrile, 0.3mL·min -1 Gradient elution. The mass spectrometry method adopts electrospray ionization, positive ion multi-reaction monitoring mode, monitoring ion pairs: osimertinib m / z 500.38→71.88 and internal standard pazopanib m / z 438.21→357.18. The inventor conducted a methodological investigation in accordance with the “Guidelines for Validation of Quantitative Analysis Methods for Biological Samples” in the 2015 Chinese Pharmacopoeia, and detected the drug concentration of osimertinib in the plasma and cerebrospinal fluid of the subjects, and initially verified the applicability of the meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com