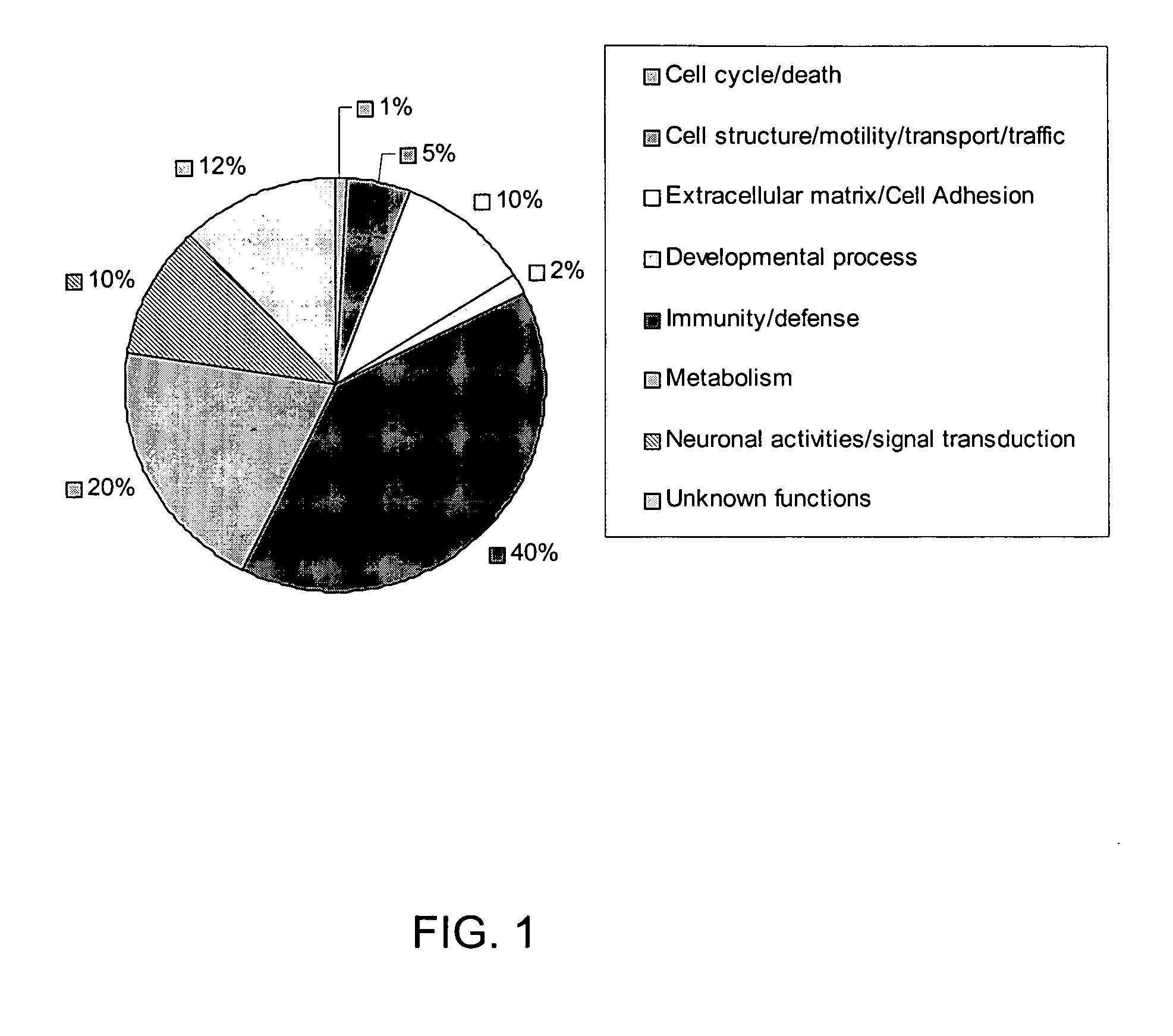
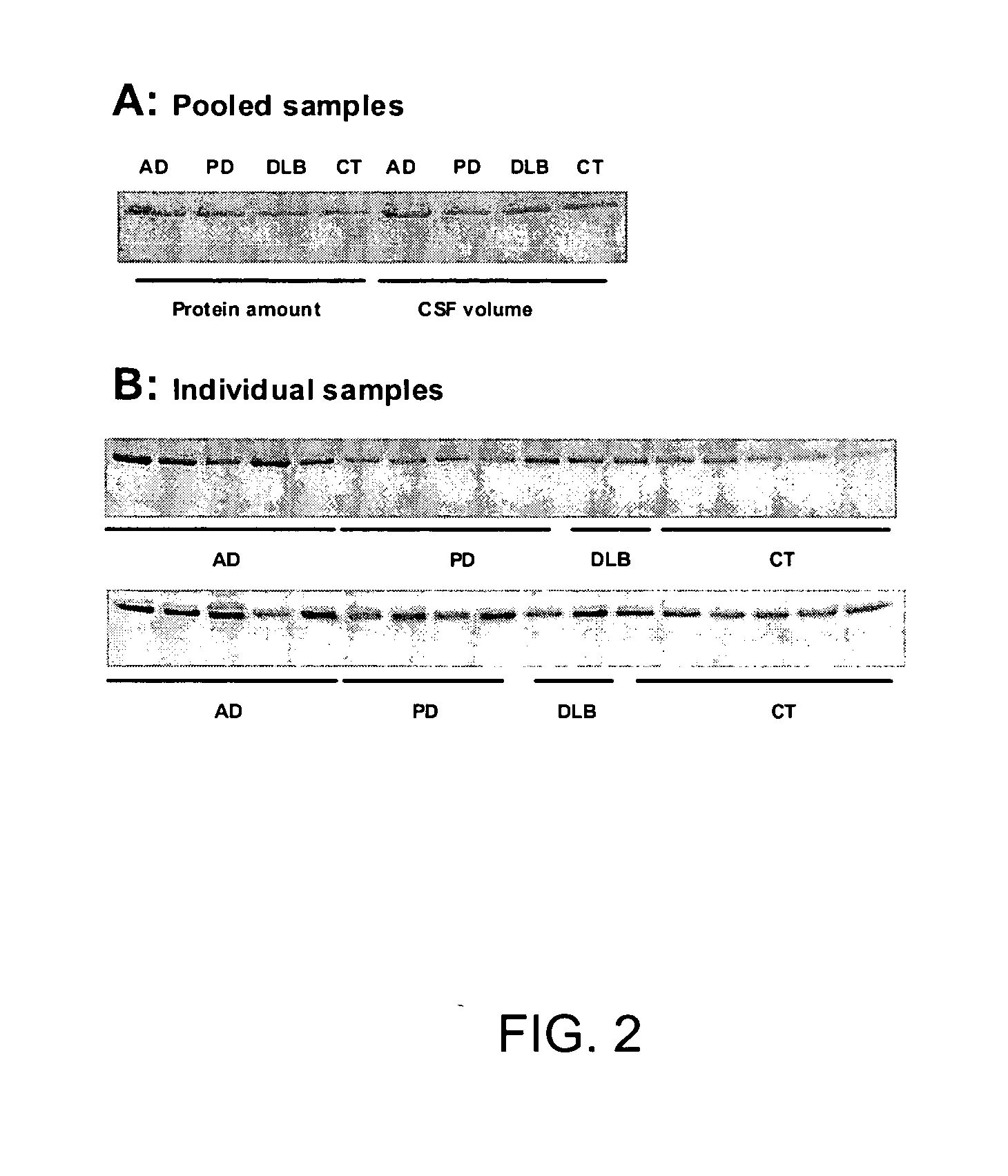
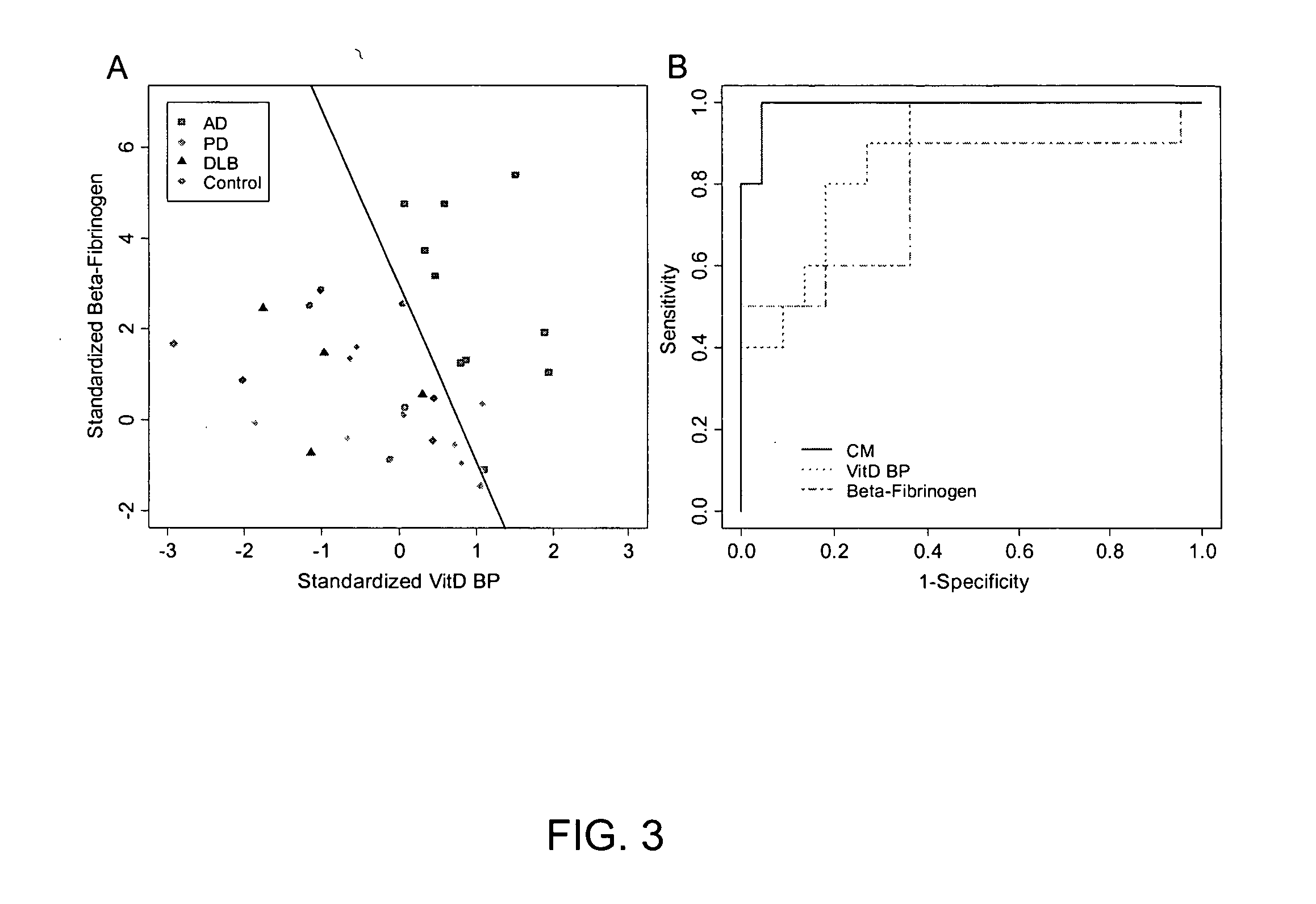
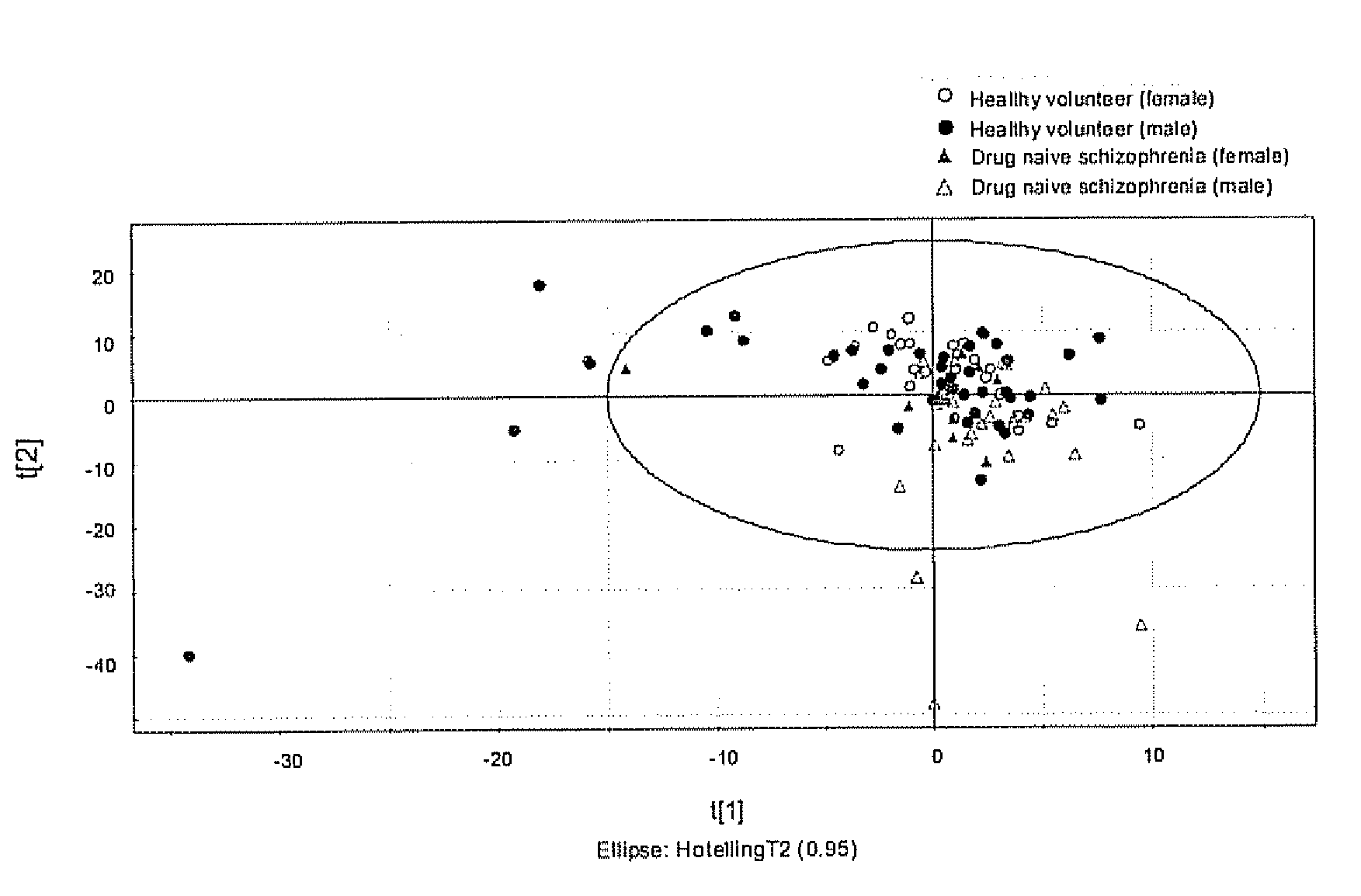
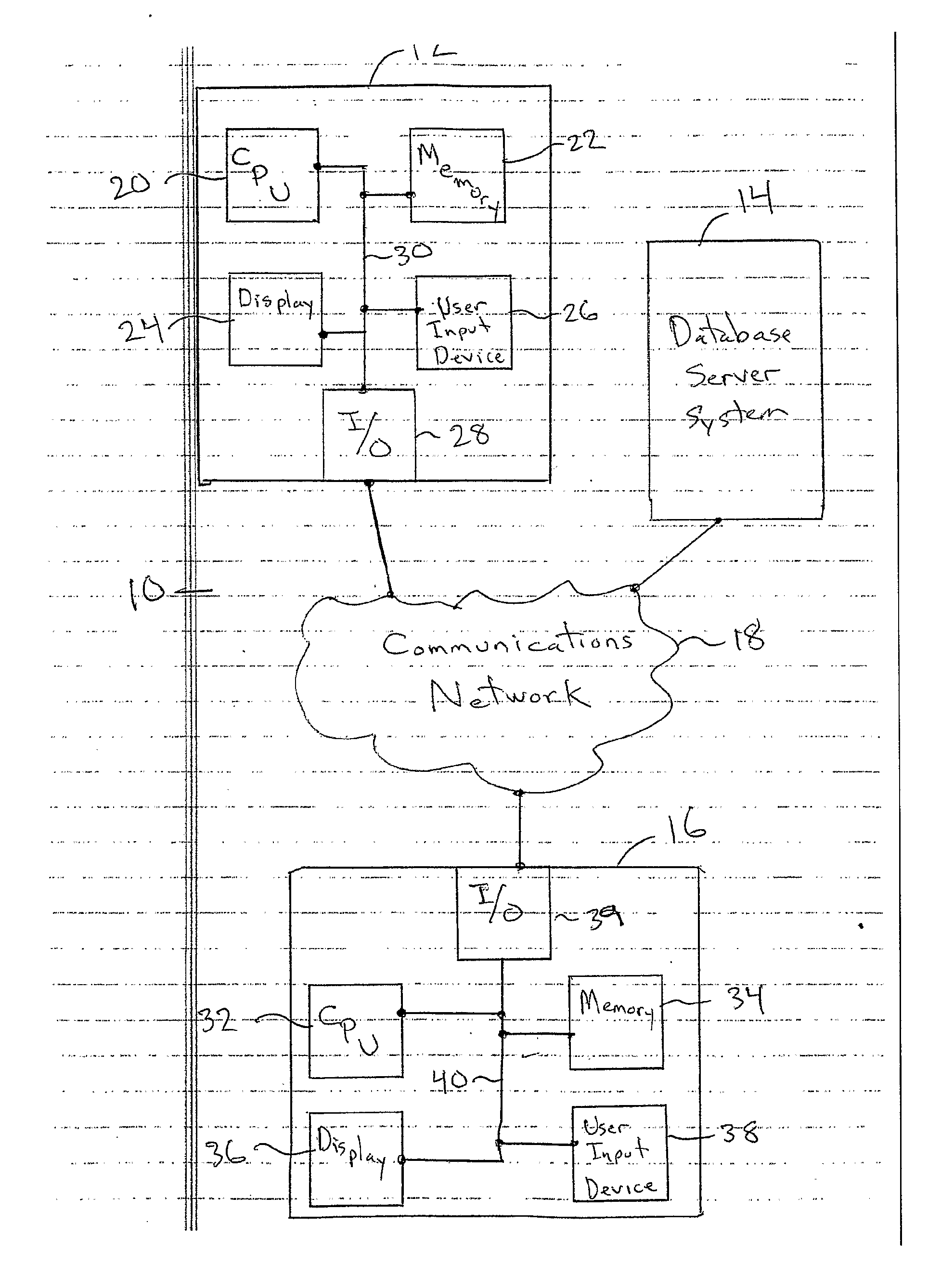
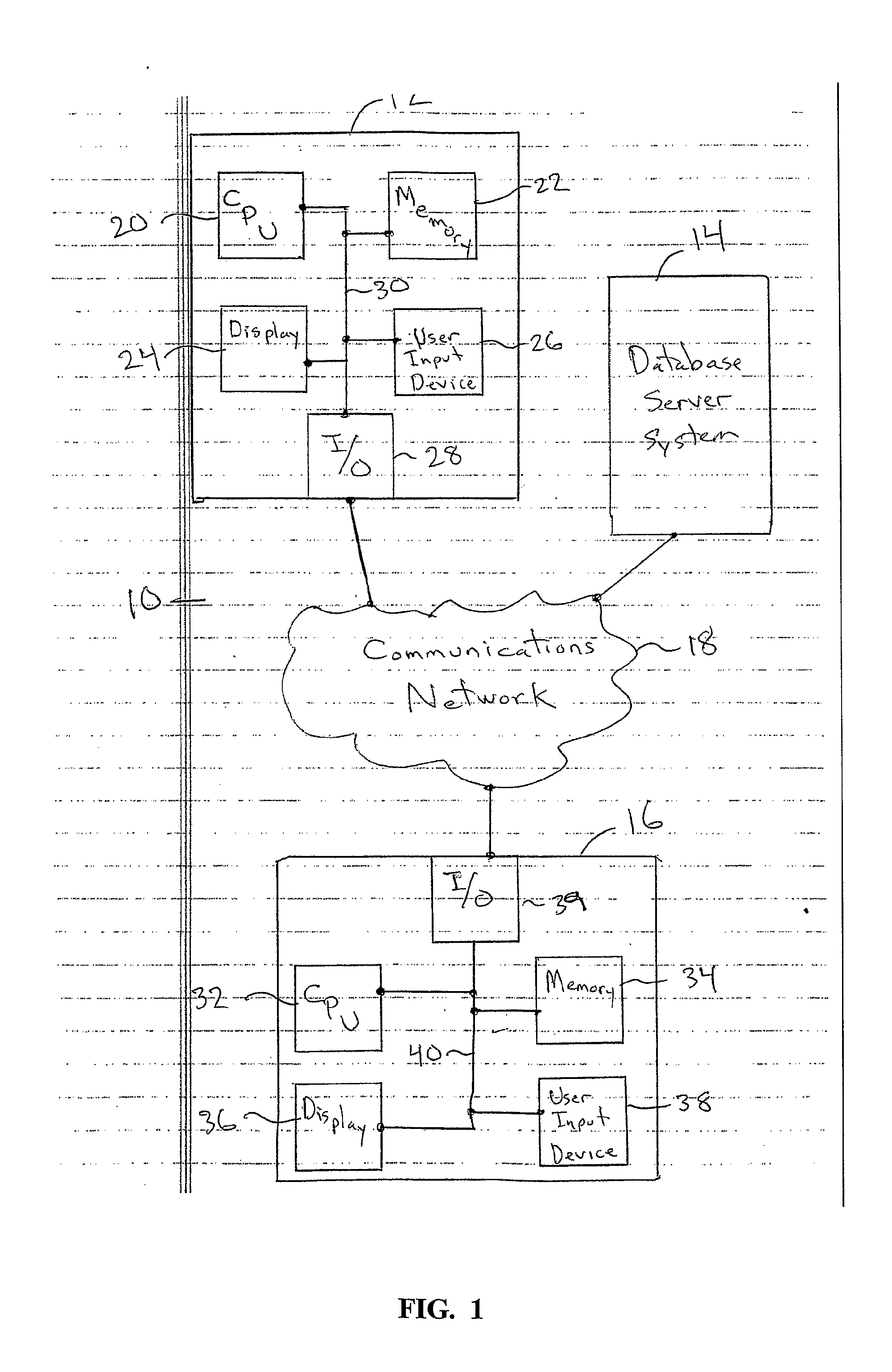
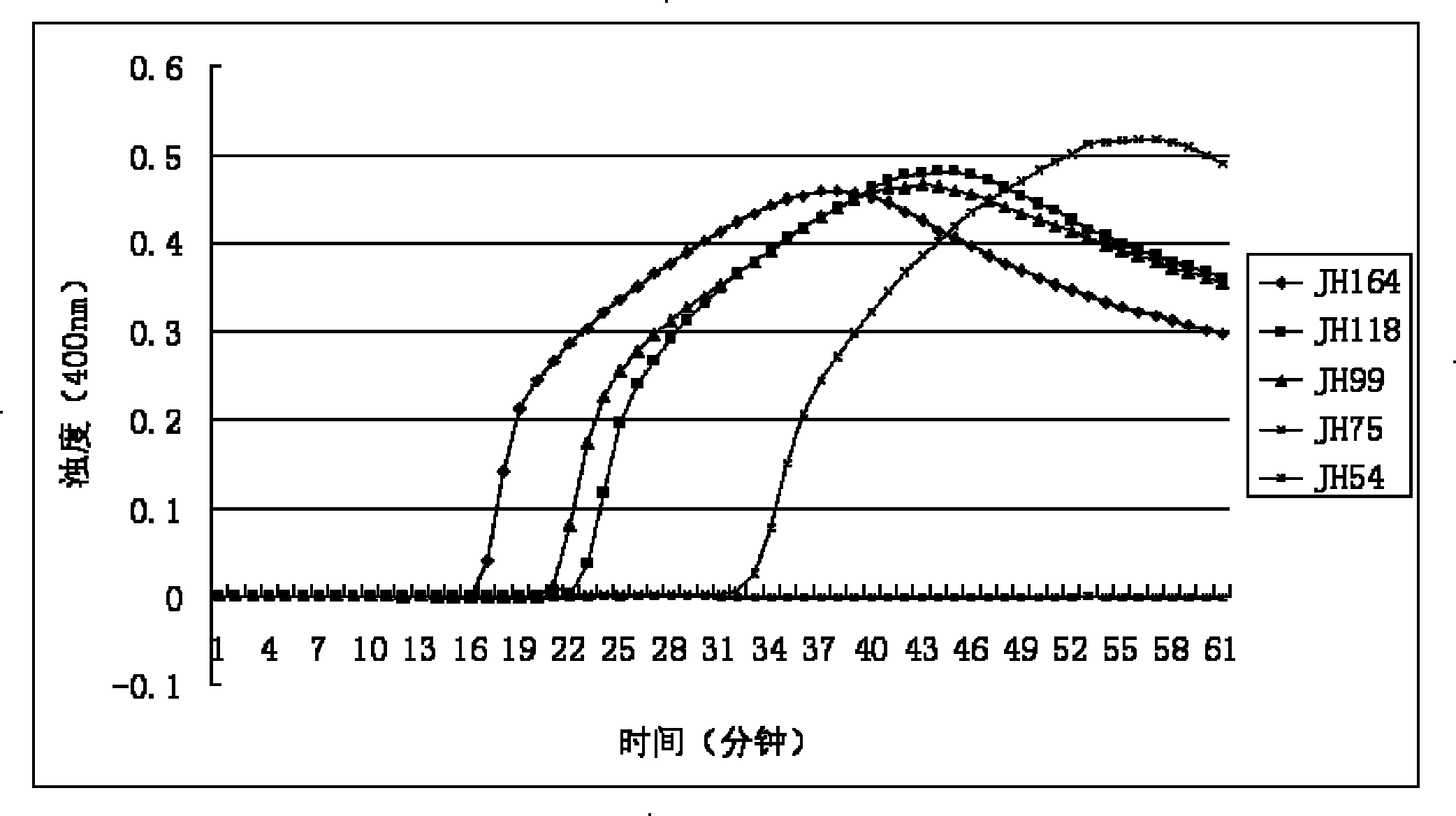
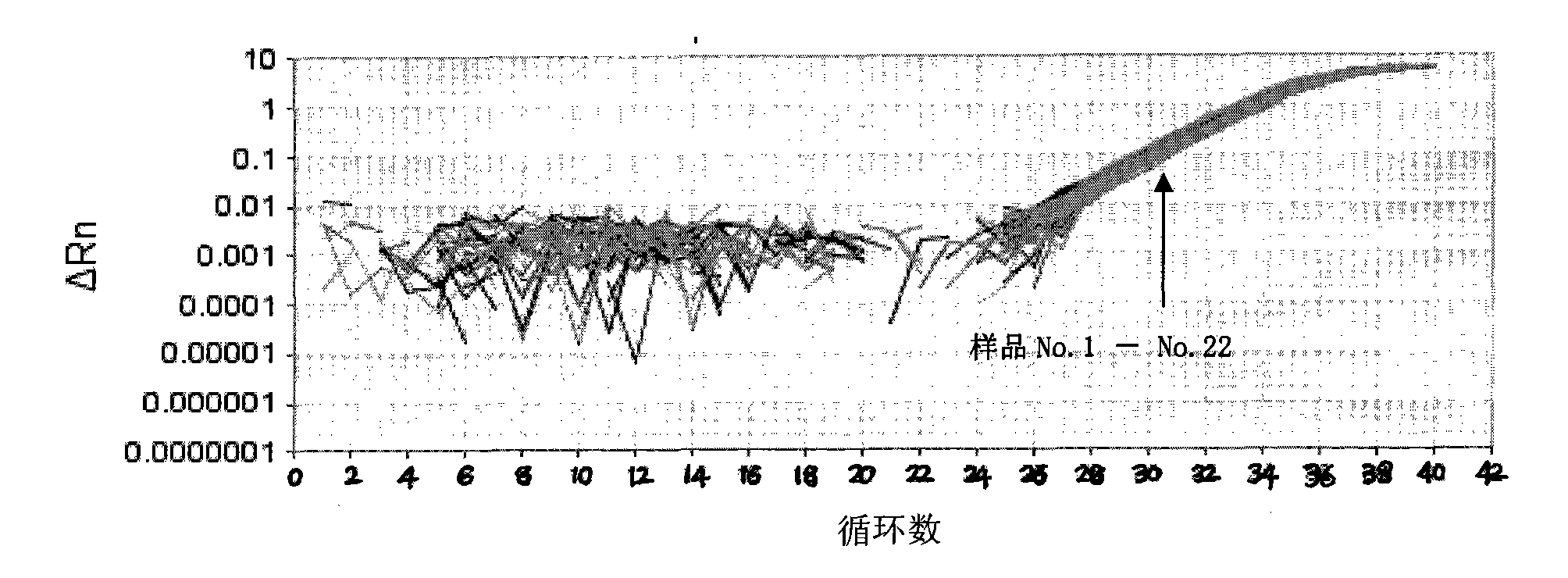
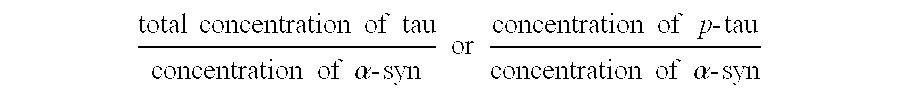Patents
Literature
52 results about "Cerebrospinal fluid sample" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cerebrospinal fluid testing is a set of laboratory analyses that contains samples of the fluid surrounding the brain and spinal cord area. Cerebral spinal fluid (CSF) is a clear watery fluid that occupies the space between the subarachnoid cavities and the pia mater. CSF acts as a cushion for the cortex while protecting the brain.
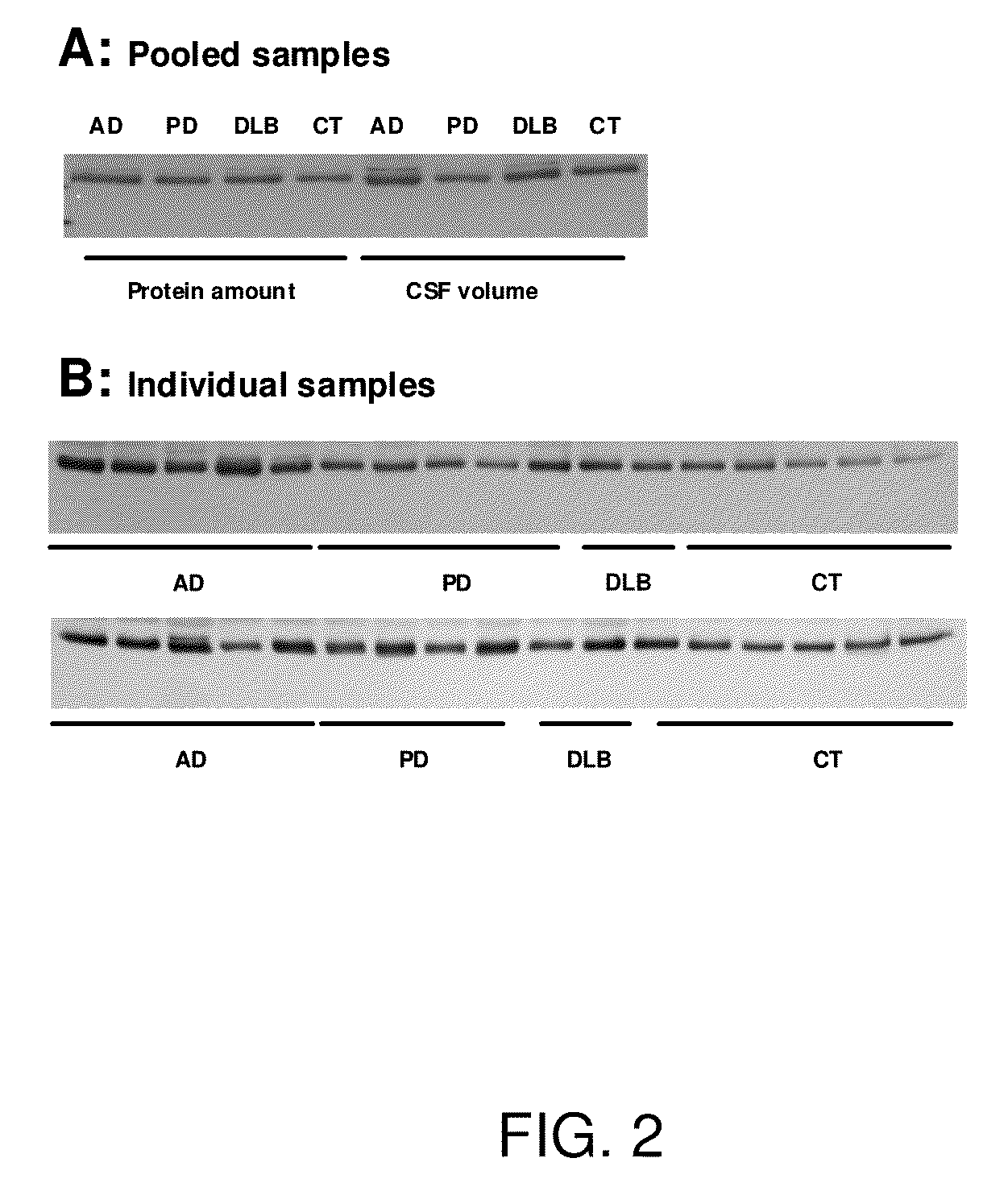
Biomarkers for neurodegenerative disorders
InactiveUS20070099203A1Medical data miningMicrobiological testing/measurementDementia with Lewy bodiesTreatment effect
The present invention provides methods for diagnosing neurodegenerative disease, such as Alzheimer's Disease, Parkinson's Disease, and dementia with Lewy body disease by detecting a pattern of gene product expression in a cerebrospinal fluid sample and comparing the pattern of gene product expression from the sample to a library of gene product expression pattern known to be indicative of the presence or absence of a neurodegenerative disease. The methods also provide for monitoring neurodegenerative disease progression and assessing the effects of therapeutic treatment. Also provided are kits, systems and devices for practicing the subject methods.
Owner:UNIV OF WASHINGTON
Biomarkers
InactiveUS20080220530A1Minimal sample preparationSuppress water NMR resonanceMagnetic measurementsMicrobiological testing/measurementDiseaseBipolar mood disorder
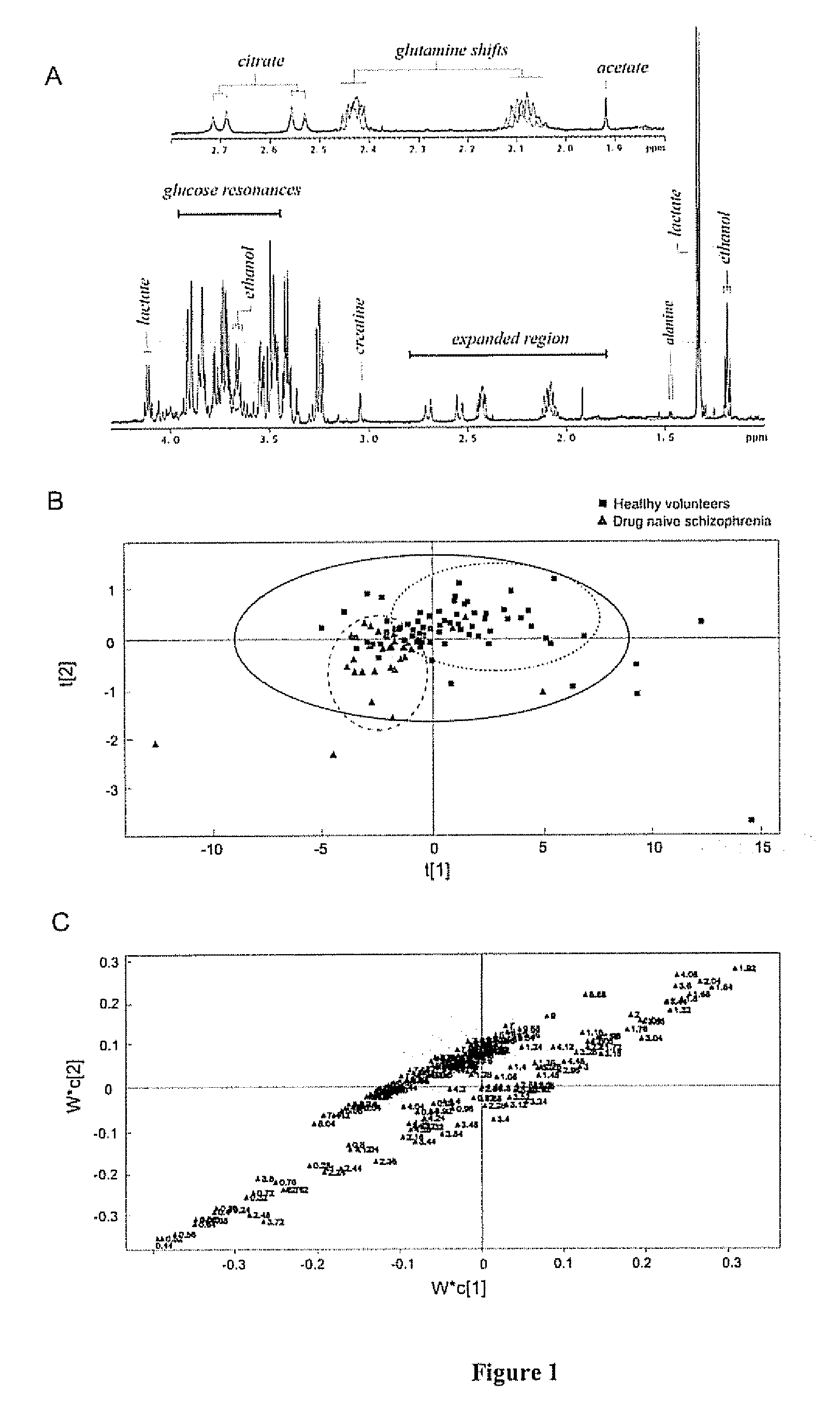
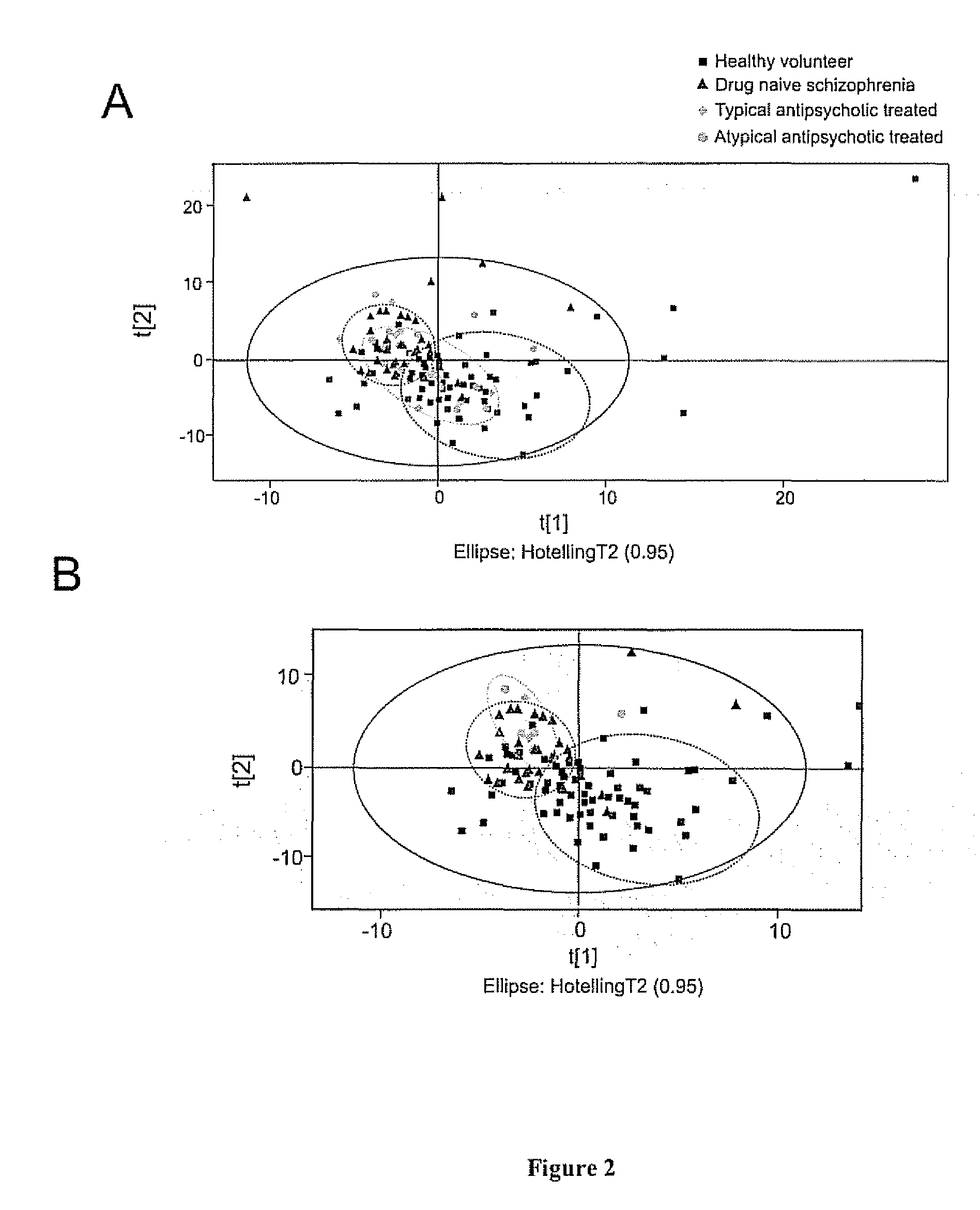
The invention relates to methods for diagnosing or monitoring psychotic disorders such as schizophrenic or bipolar disorders, comprising measuring the level of one or more biomarker(s) present in a cerebrospinal fluid sample taken from a test subject, said biomarker(s) being selected from the group consisting of: glucose, lactate, acetate species and pH. The invention also relates to methods of diagnosing or monitoring a psychotic disorder in a subject comprising providing a test sample of CSF from the subject, performing spectral analysis on said CSF test sample to provide one or more spectra, and, comparing the one or more spectra with one or more control spectra. The invention also relates to sensors, biosensors, multi-analyte panels, arrays, assays and kits for performing methods of the invention.
Owner:PSYNOVA NEUROTECH LTD
Biomarkers for neurodegenerative disorders
Owner:UNIV OF WASHINGTON
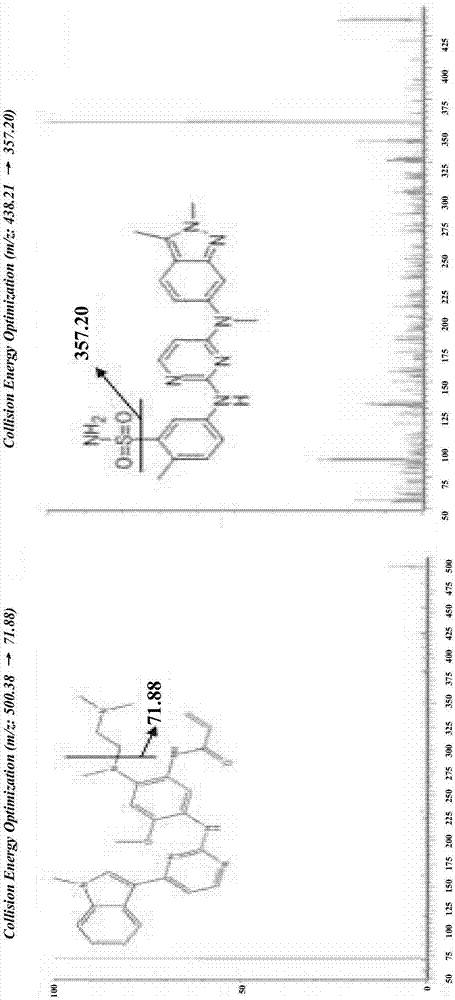
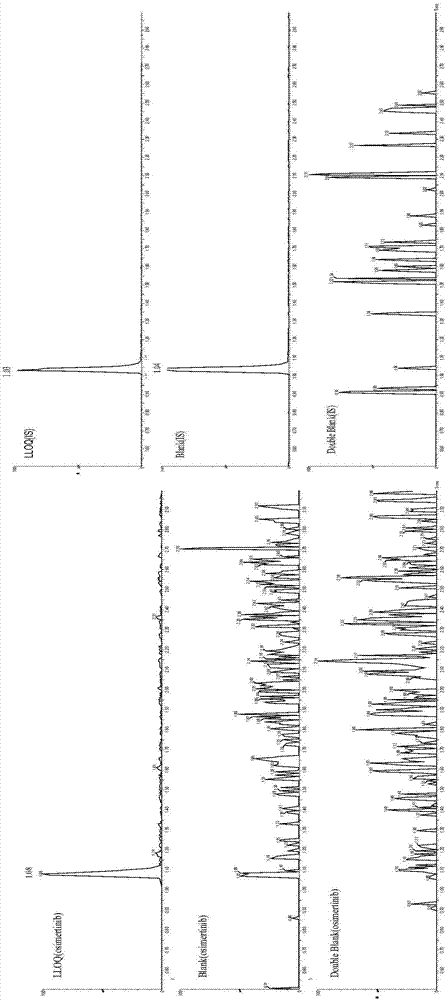
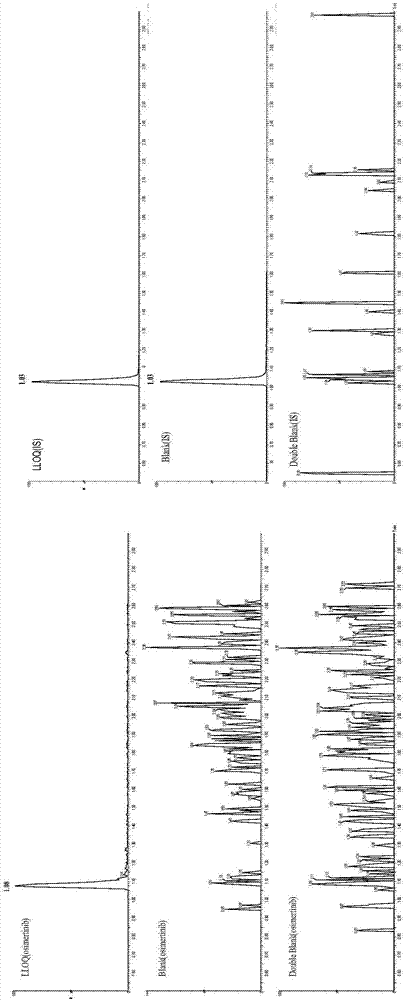
Detection of drug concentration of osimertinib in human plasma and/or cerebrospinal fluid by combining UPLC-MS/MS
The invention relates to detection of the drug concentration of osimertinib in human plasma and / or cerebrospinal fluid by combining UPLC-MS / MS. According to the present invention, the method for determining the drug concentration of osimertinib in human plasma and / or cerebrospinal fluid through an ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS / MS) is provided, wherein formic acid-containing ammonium formate water-formic acid acetonitrile is used as a mobile phase so as to perform the method; and the method has advantages of good specificity and high sensitivity, and is used for the detection of clinical pharmacokinetic samples, wherein the linearity is good when the osimertinib concentration is 2-500 ng.ml<-1> in the plasma sample and the osimertinib concentration is 0.5-20 ng.ml<-1> in the cerebrospinal fluid sample.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Multiplexed biomarkers for monitoring the alzheimer's disease state of a subject
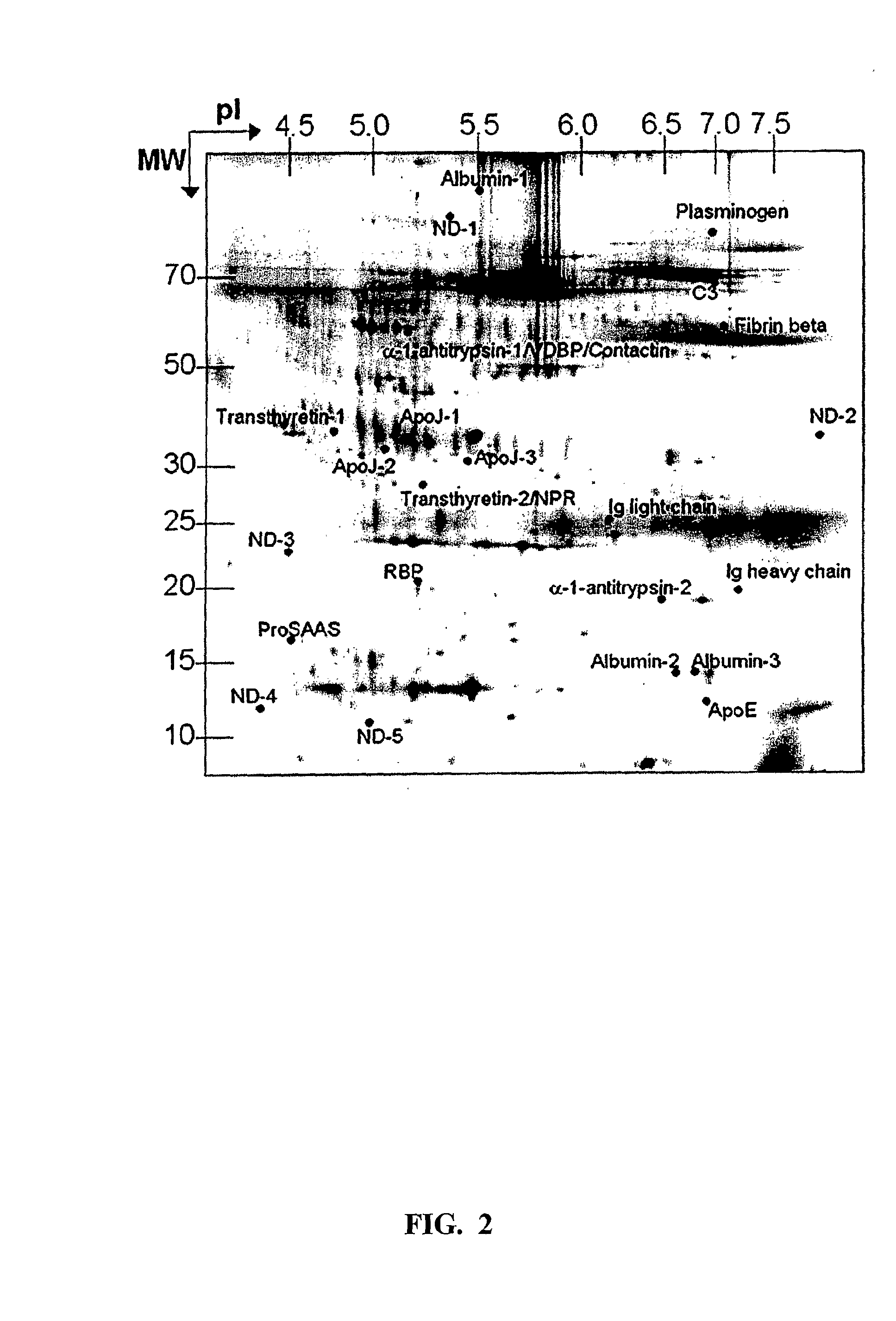
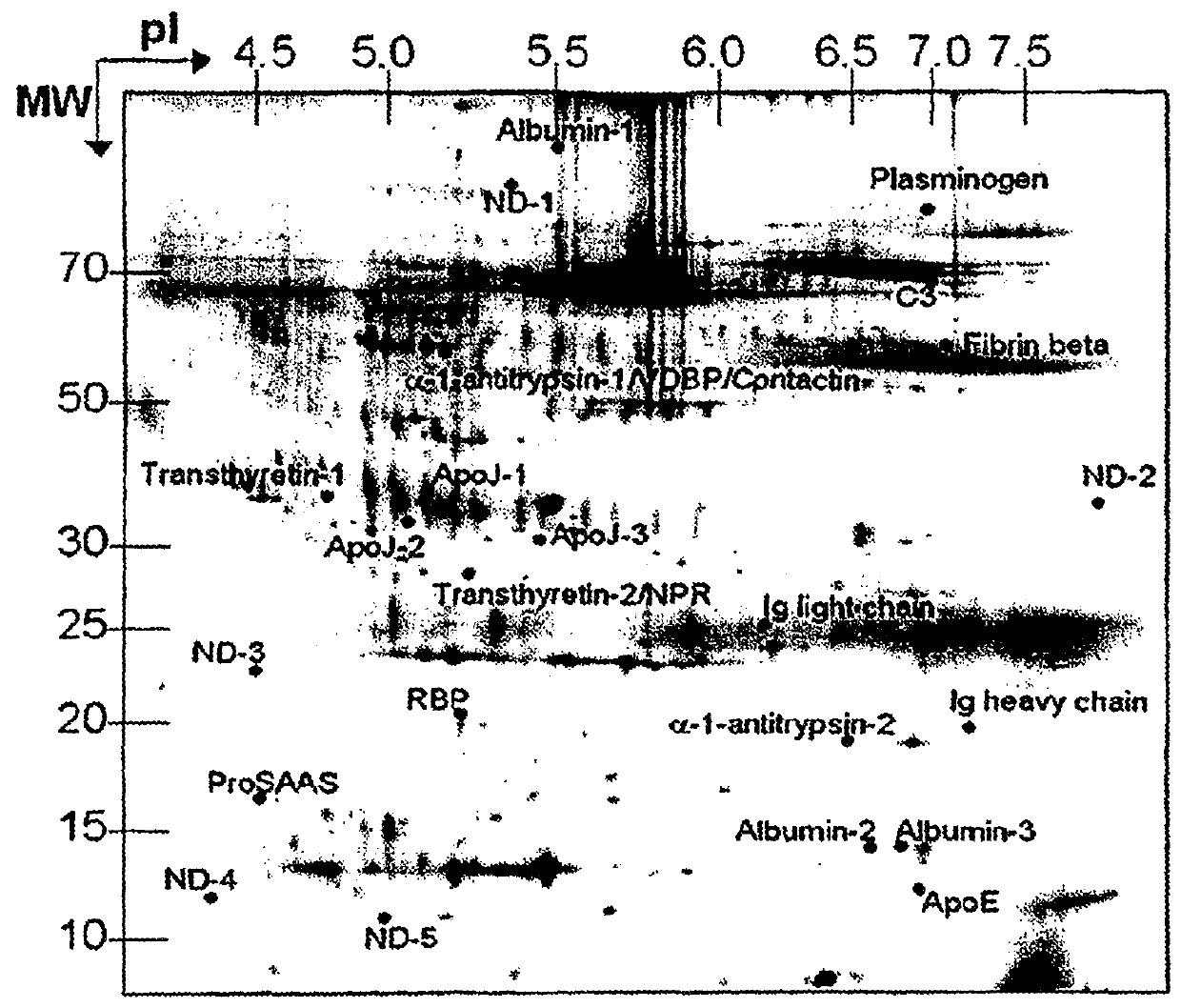
The present invention relates to a method for diagnosing a subject's Alzheimer's disease state. The method involves providing a database containing information relating to protein expression levels associated and not associated with Alzheimer's disease. The database includes information relating to at least a majority of the following proteins: albumin, alpha-1-antitrypsin, apolipoprotin E, apolipoprotein J, complement component 3, contactin, fibrin beta, Ig heavy chain, Ig light chain, neuronal pentraxin receptor, plasminogen, proSAAS, retinol-binding protein, transthyretin, and vitamin D binding protein. Information relating to proteins found in one or more cerebrospinal fluid samples from a subject is also provided and a database is used to analyze the information from the subject to diagnose the subject's Alzheimer's disease state. Also disclosed is a computer readable medium and a system, both useful in carrying out the present invention.
Owner:CORNELL RES FOUNDATION INC
Loop-mediated isothermal amplification (LAMP) detection method for mycobacterium tuberculosis, and special primer and kit thereof
ActiveCN102399901AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseLoop-mediated isothermal amplification
The invention discloses a loop-mediated isothermal amplification (LAMP) detection method for mycobacterium tuberculosis, and a special primer and a kit thereof. The special primer contains a primer combination consisting of six primers, namely a forward inner primer (FIP), a backward inner primer (BIP), a left forward (LF) primer, a left backward (LB) primer, an F3 primer and a B3 primer. The LAMP detection method can be used for quickly, conveniently and efficiently detecting the mycobacterium tuberculosis with high specificity and high sensitivity under the isothermal condition, does not require complex instruments, and can be used for detecting pure bacteria samples, samples of clinical sputum and cerebrospinal fluid and the like. The method provides a new technical platform for detecting the mycobacterium tuberculosis, and can be used for screening and detecting the mycobacterium tuberculosis in basic-level medical health units and disease prevention and control centers.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Rapid nucleic acid extraction kit and applications thereof
The invention belongs to the technical field of biological nucleic acid detection, and particularly relates to a rapid nucleic acid extraction kit and applications thereof. The kit of the invention comprises a grinding rod, an extraction liquid, an extraction column, a micro ultra-filter and a centrifuge tube, wherein the extraction liquid is a DEPC aqueous solution containing chelex-100. The invention further relates to a use method for the kit, and applications of the kit in extractions of DNA or RNA from pathological tissue paraffin section samples, samples of blood stains and semen stains, tissue fluid samples, cerebrospinal fluid samples, blood samples, serum samples, plasma samples, urine samples, hydrops samples, and tissue cell samples. According to the present invention, content and quality of the nucleic acid extracted by the kit of the present invention can well meet requirements of follow-up reactions of PCR, RT-PCR and sequencing. With the present invention, limitation of complexity and time consuming of nucleic acid extraction in the prior art are overcome well, and characteristics of safety and environmental protection are provided.
Owner:上海源奇生物医药科技有限公司
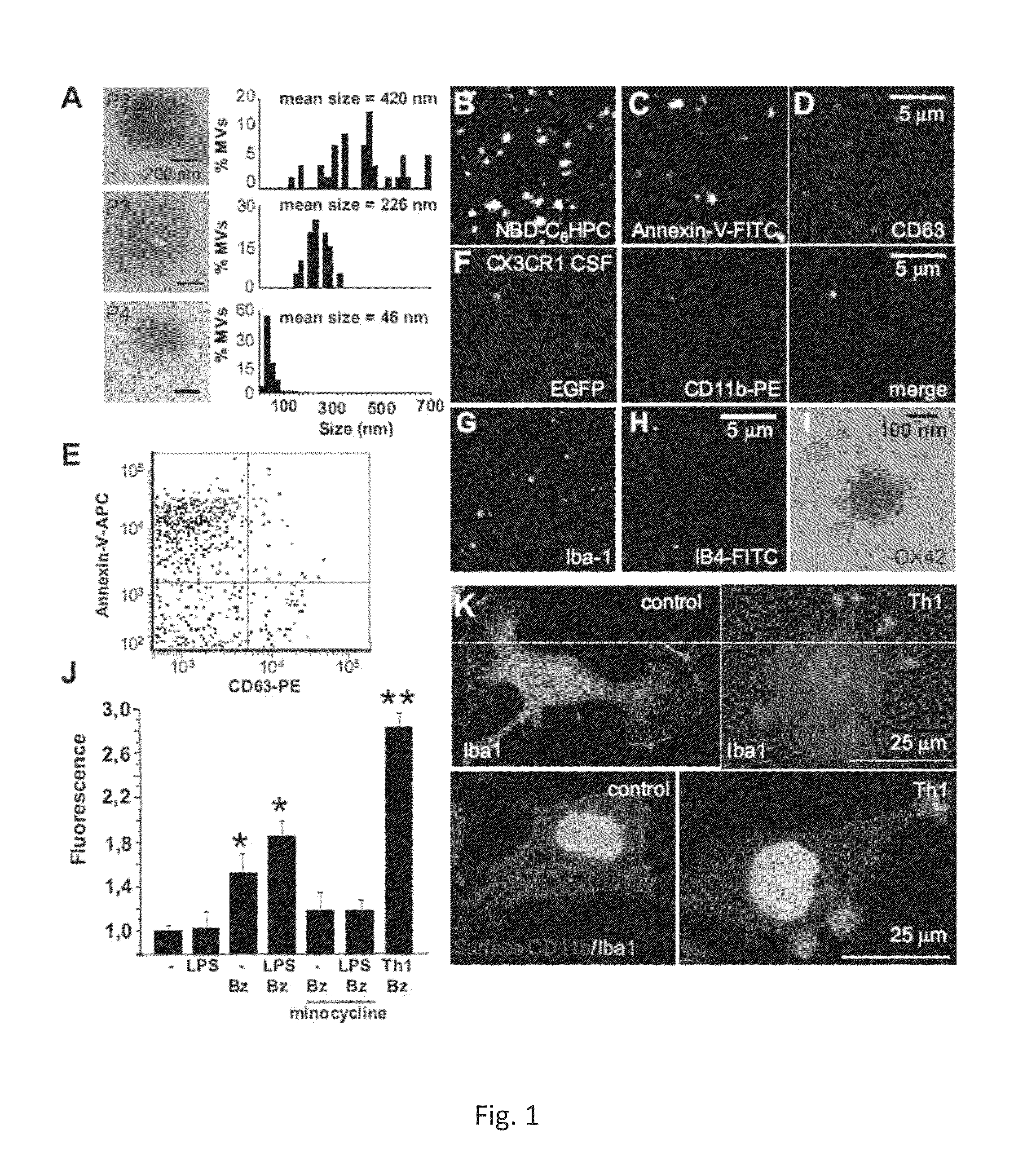
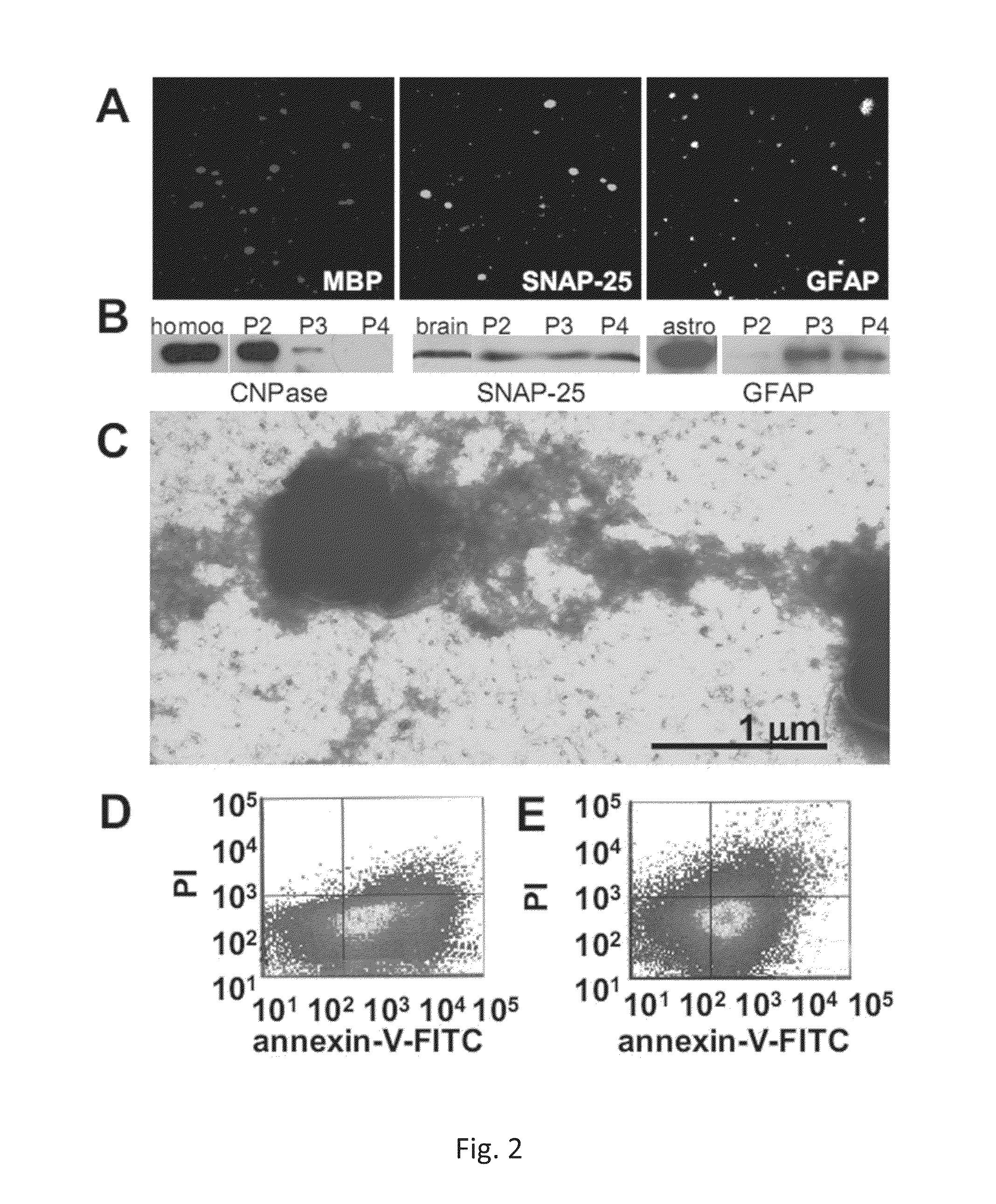
Increase of myeloid microvesicles in the cerebrospinal fluid as biomarker of microglia/macrophage activation in neurological disorders
InactiveUS8999655B2Microbiological testing/measurementLibrary screeningInflammation ProcessNervous system
The present invention relates to a method for the diagnostic and / or prognostic of a neurological disease characterized by an inflammation process in a subject comprising measuring the amount of myeloid derived microvesicles in a cerebrospinal fluid sample obtained from the subject. The invention further relates to a method for predicting and / or monitoring the efficacy of a treatment for a neurological pathology or for monitoring a neurological disease progression.
Owner:OSPEDALE SAN RAFFAELE SRL +2
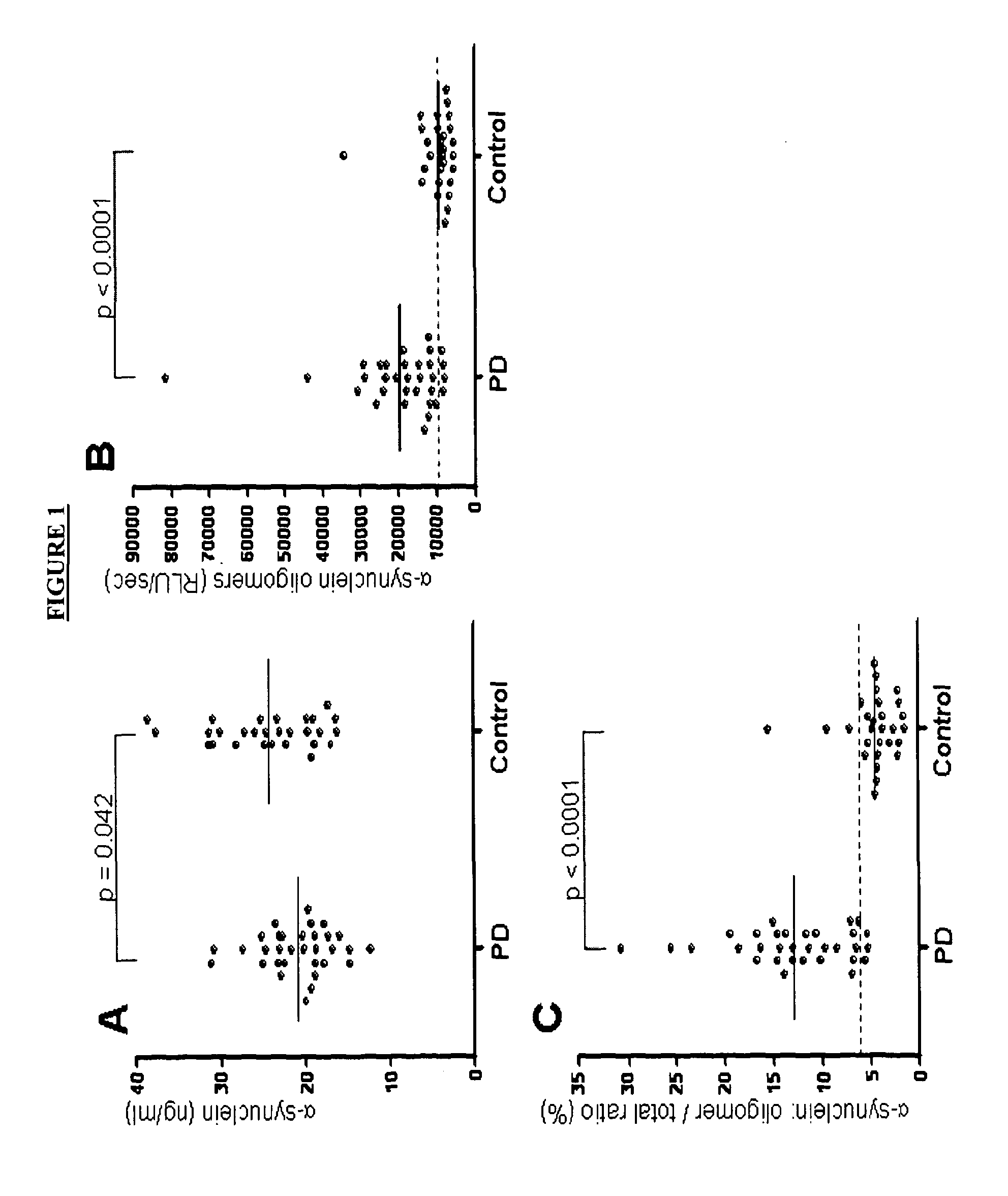
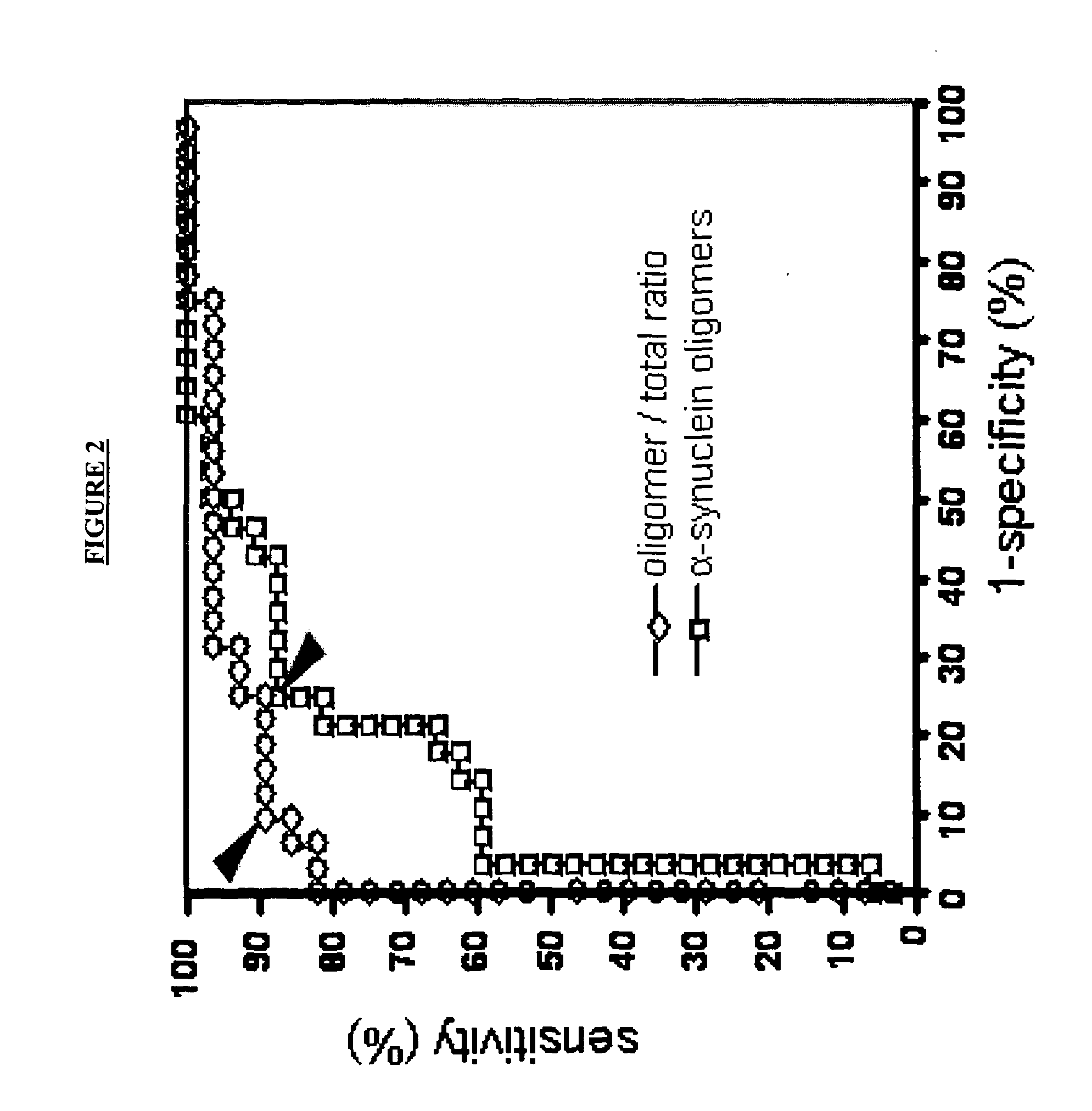
Diagnostic agent for parkinson's disease
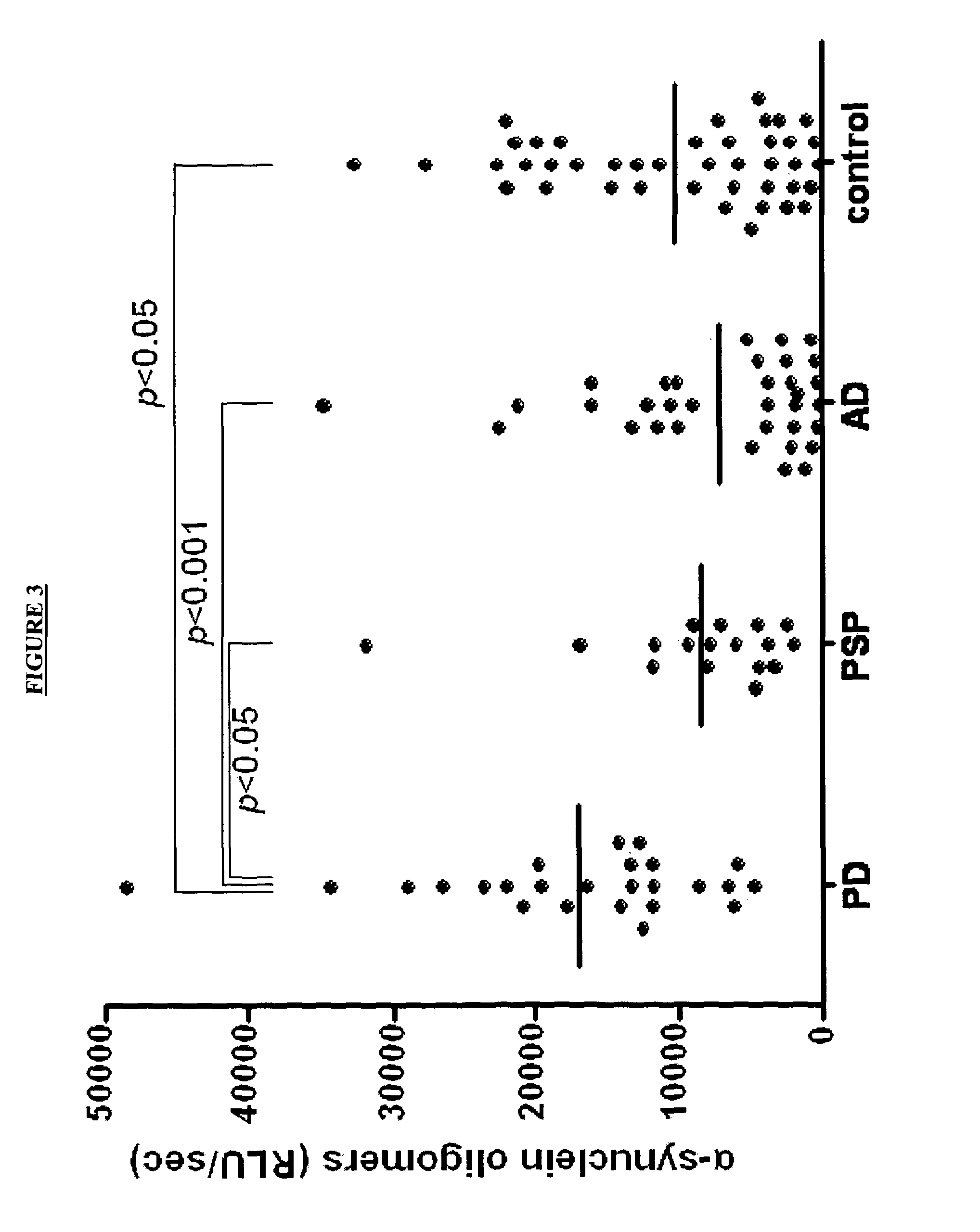
The present invention relates to method of identifying whether or not an individual has Parkinson's disease (PD). In particular, the invention relates to a method for identifying whether or not an individual has PD in the pre-symptomatic phase of the disease or to distinguishing PD from another neurological disorder. The method of the invention comprises measuring the amount of soluble α-synuclein oligomers in a cerebrospinal fluid sample taken from an individual. The method optionally also comprises measuring the total amount of α-synuclein in the CSF sample, calculating the ratio of the amount of α-synuclein oligomers to the total amount of α-synuclein, and thereby determining whether or not the individual has PD. The method of the invention can be used in clinical trials to measure the effect of drugs in both PD animal models and human PD patients.
Owner:UNITED ARAB EMIRATES UNIVERSITY
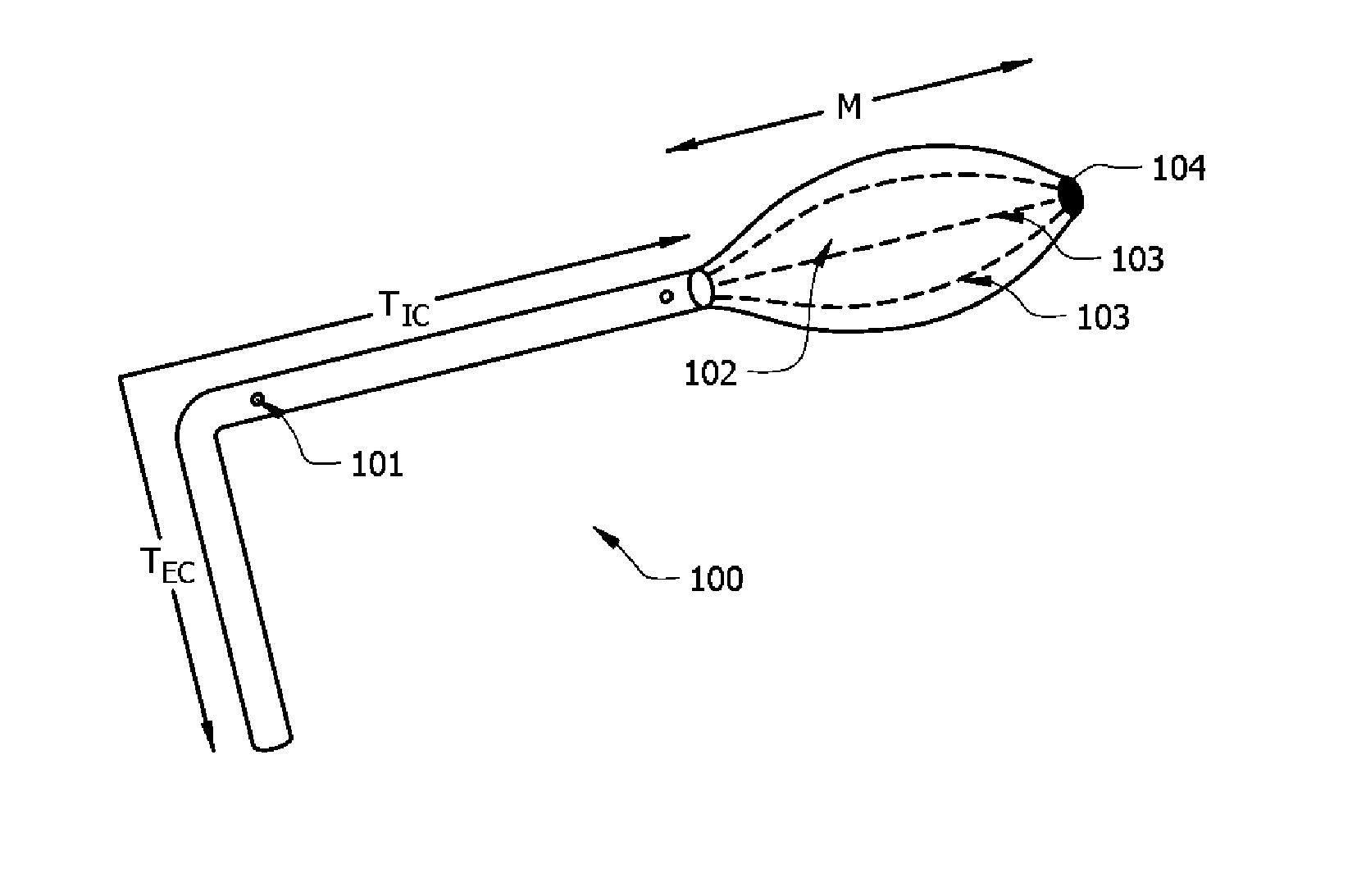
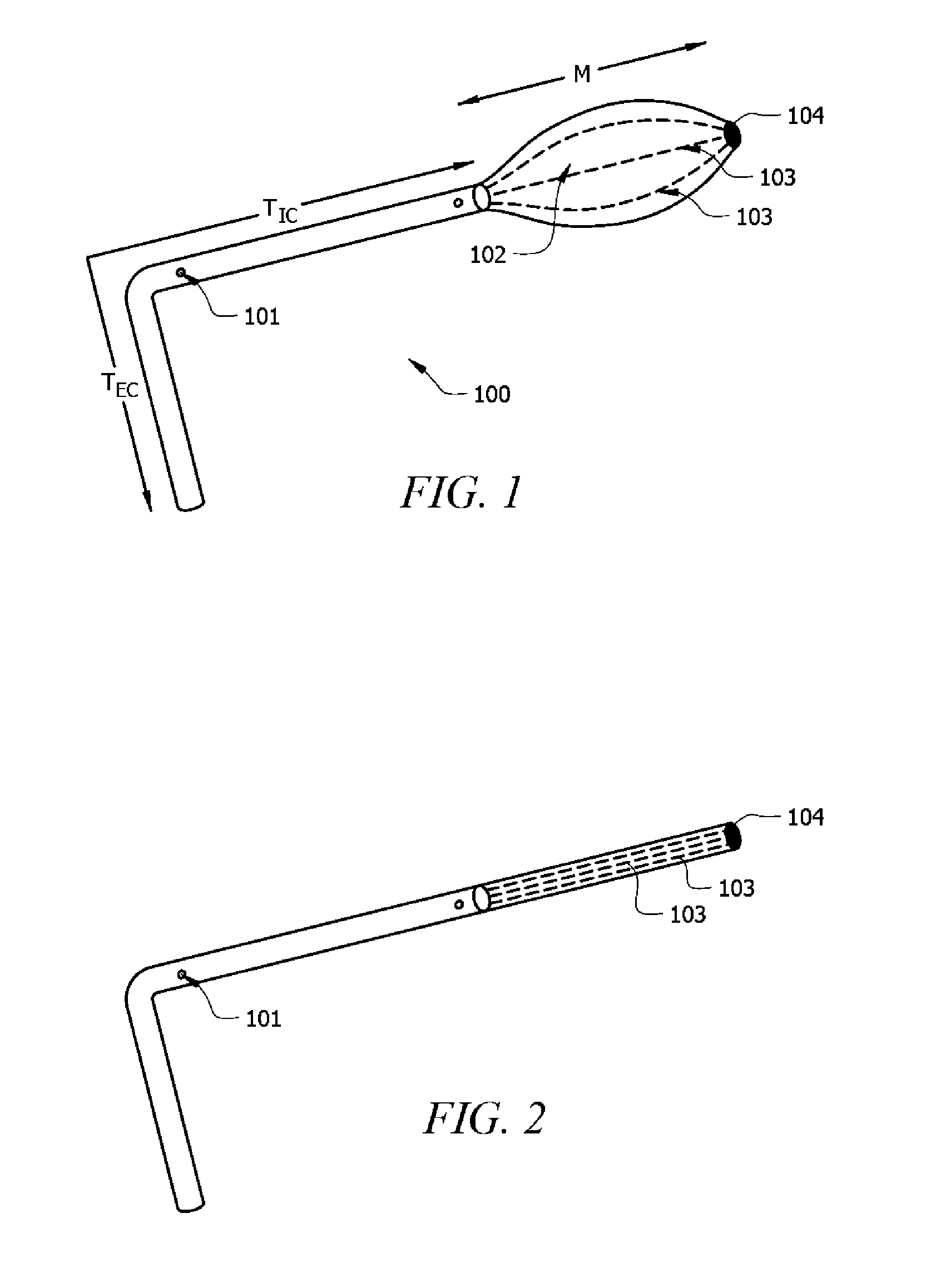
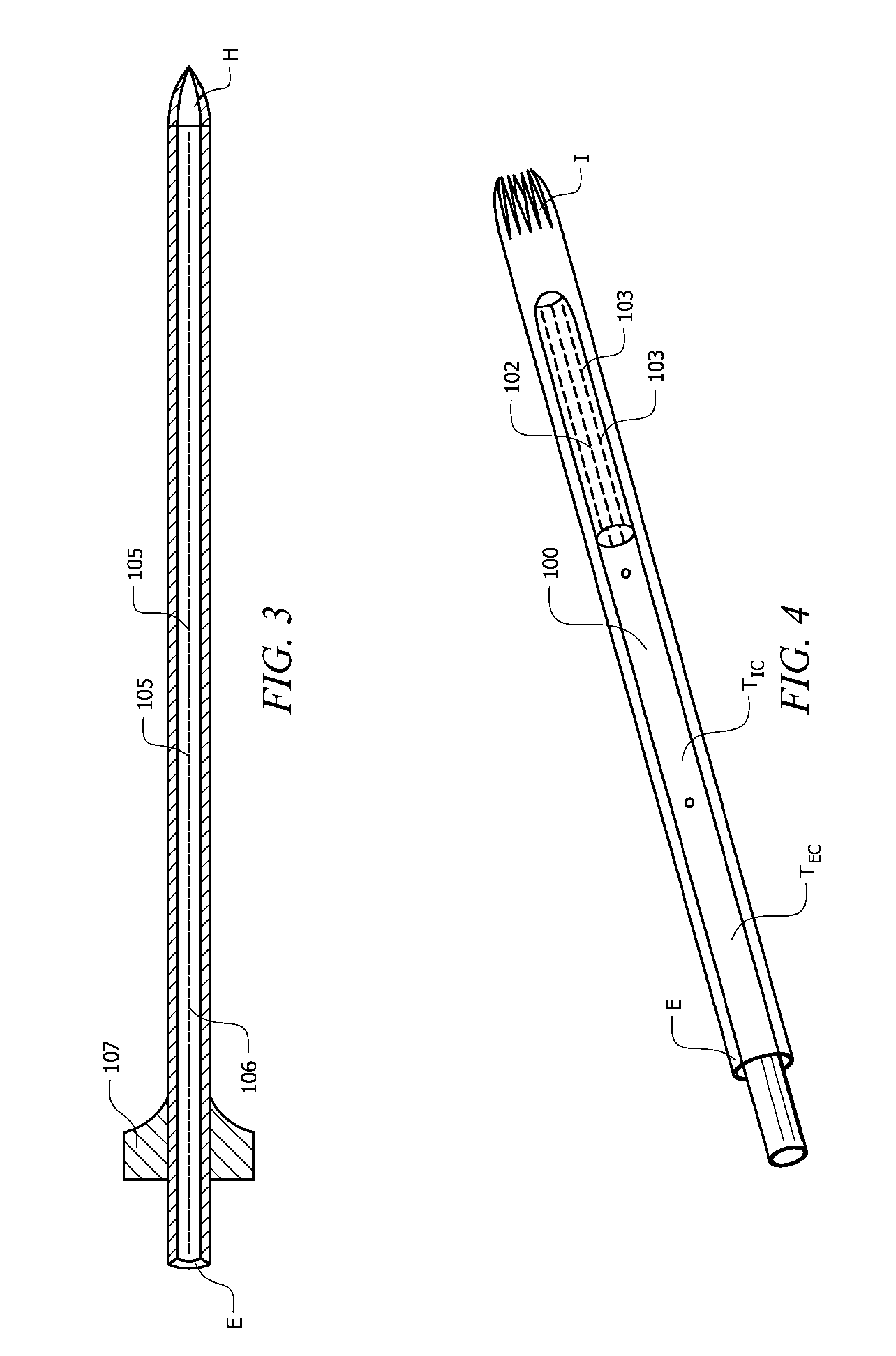
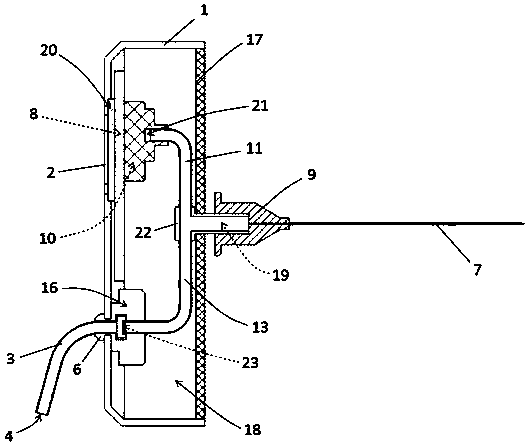
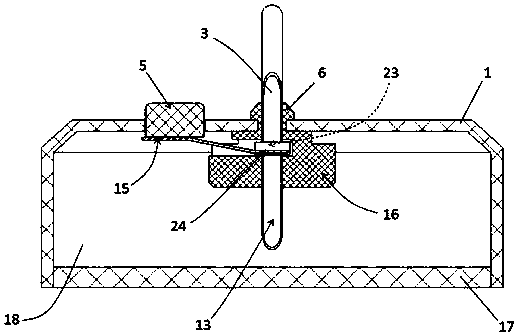
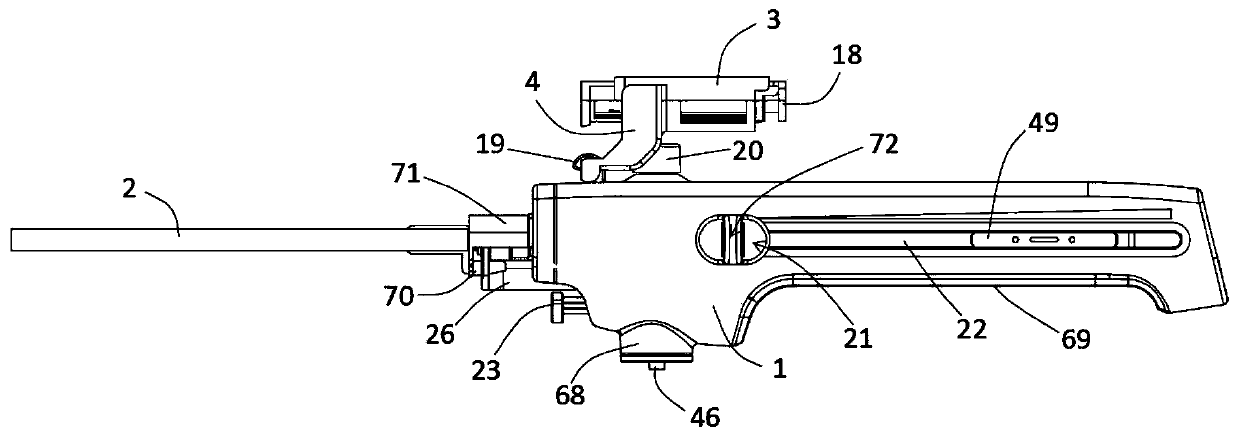
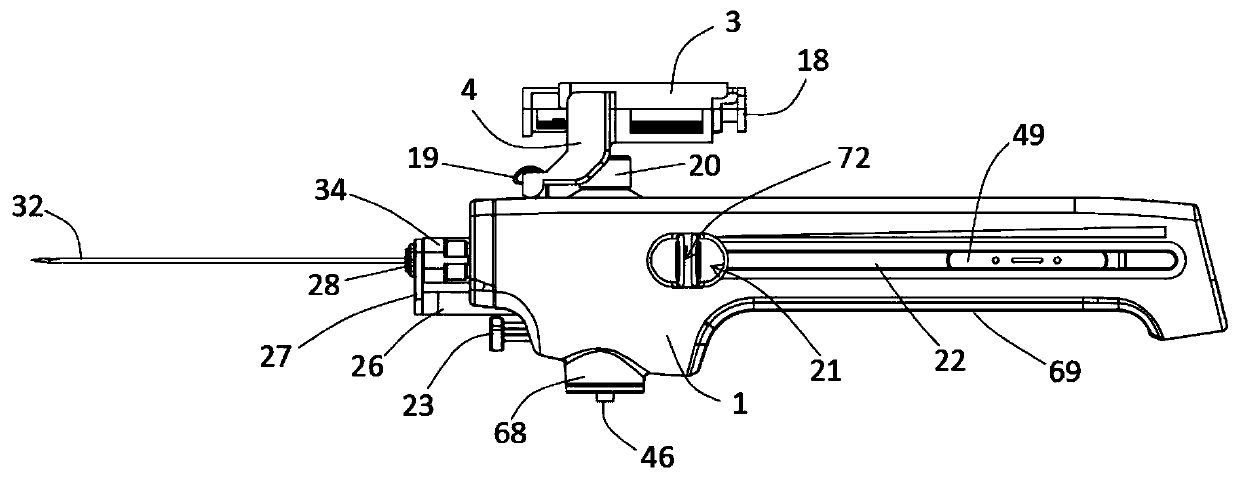
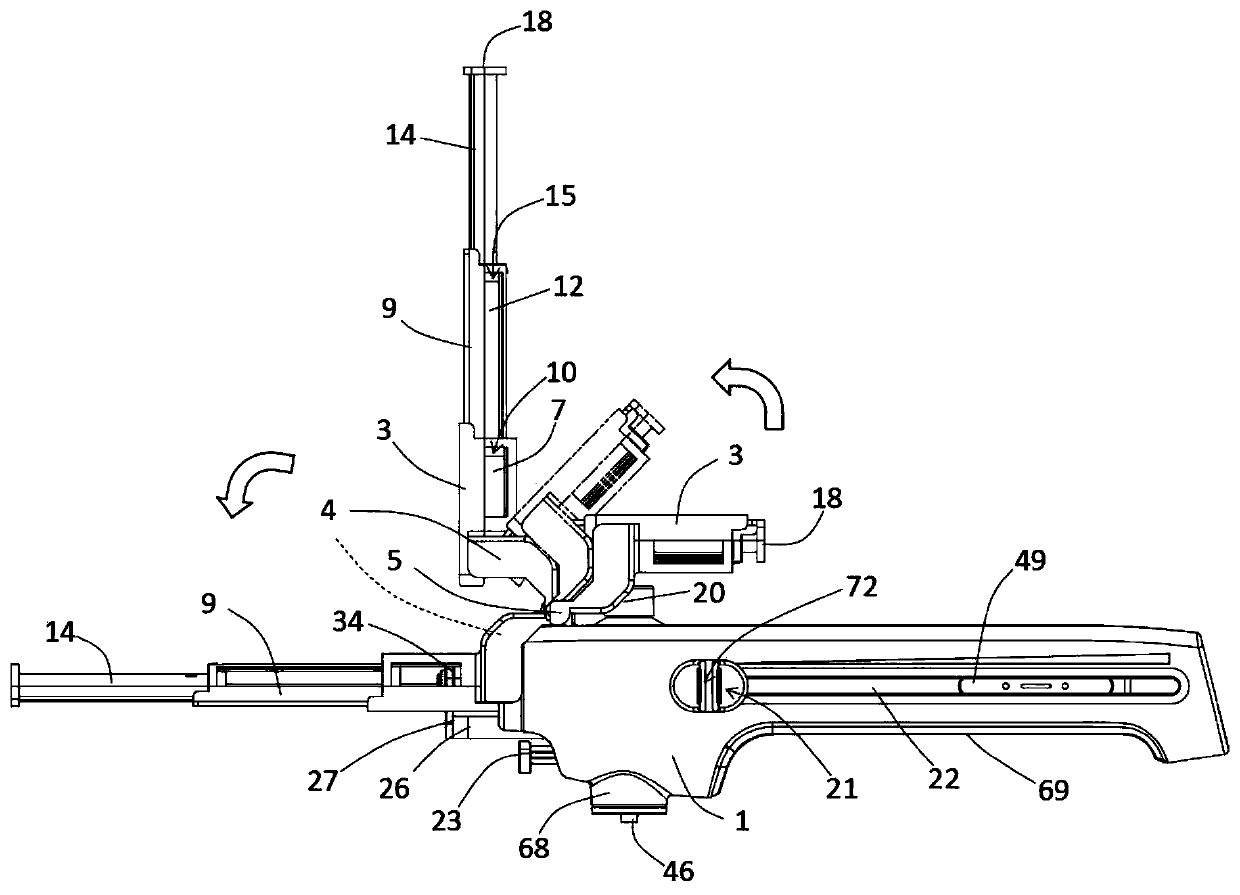
Integrated safe lumbar puncture device
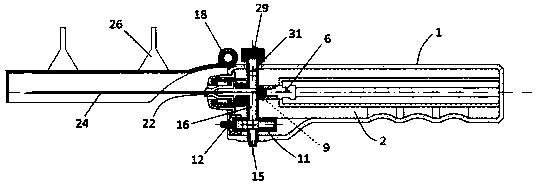
ActiveCN108338825AGuarantee the safety of lifeQuick pull outSurgical needlesVaccination/ovulation diagnosticsCerebrospinal fluid pressureCerebral hernia
The invention discloses an integrated safe lumbar puncture device used clinically. The integrated safe lumbar puncture device is mainly applied to the conditions that cerebrospinal fluid puncture needs to be conducted in the clinical diagnosis and treatment process, for example, the cerebrospinal fluid pressure test is conducted during lumbar puncture as well as a cerebrospinal fluid sample is remained to perform laboratory examination. The integrated safe lumbar puncture device is characterized in that the traditional lumbar puncture needle can be replaced; the cerebrospinal fluid pressure test can be realized in the same device; the cerebrospinal fluid sample is remained according to the requirement and intrathecal injection is conducted; cerebral hernia caused by high cranial pressure excessive drainage in the lumbar puncture process can be avoided; the operation processes of sample retention, pressure test and intrathecal injection can be completed in the same device; the operationflow is simplified; puncture complications are avoided; and the safety of patients and operators is guaranteed.
Owner:迈德微创(天津)医疗器械有限责任公司
Constructing method of central system infection pathogen detection library based on high-throughput sequencing, detection method and kit
PendingCN109610008AUnbiasedSolve the problem of high build failure rateMicrobiological testing/measurementLibrary creationFailure ratePolyethylene glycol
The invention discloses a constructing method of a central system infection pathogen detection library based on high-throughput sequencing, a detection method and a kit. The library construction method comprises the following steps: extracting deoxyribonucleic acid (DNA) from a sample, and fragmenting the DNA to obtain a segmented DNA sequence; adding the segmented DNA sequence into a tail end repairing system to carry out a tail end repairing reaction, directly adding a linker sequence, a connecting enzyme, adenosine triphosphate (ATP) and polyethylene glycol into the reaction system after the tail end repairing reaction is completed, taking the ATP as an auxiliary enzyme under the action of the connecting enzyme to catalyze the linker sequence to be connected to two ends of the product of the tail end repairing reaction, so that a linker connecting product is obtained; and purifying the linker connecting product, and adding a nucleic acid single chain which is complementary to two ends of the linker sequence and used as a primer for polymerase chain reaction (PCR) amplification. The method can be used to realize rapid and broad-spectrum central system infection pathogen microorganism detection, too much pre-known clinical information of detection samples is not needed, detection can be carried out quickly and efficiently, the detection result is free of deviation, and a problem that the library construction failure rate of low-concentration cerebrospinal fluid samples is high can be solved.
Owner:深圳华大因源医药科技有限公司 +1
Methods for assessing a condition by analyzing blood in cerebrospinal fluid
InactiveUS8027814B2Accurate diagnosisReliable resultsRadiation pyrometryAnalogue computers for chemical processesPoint of careAnalyte
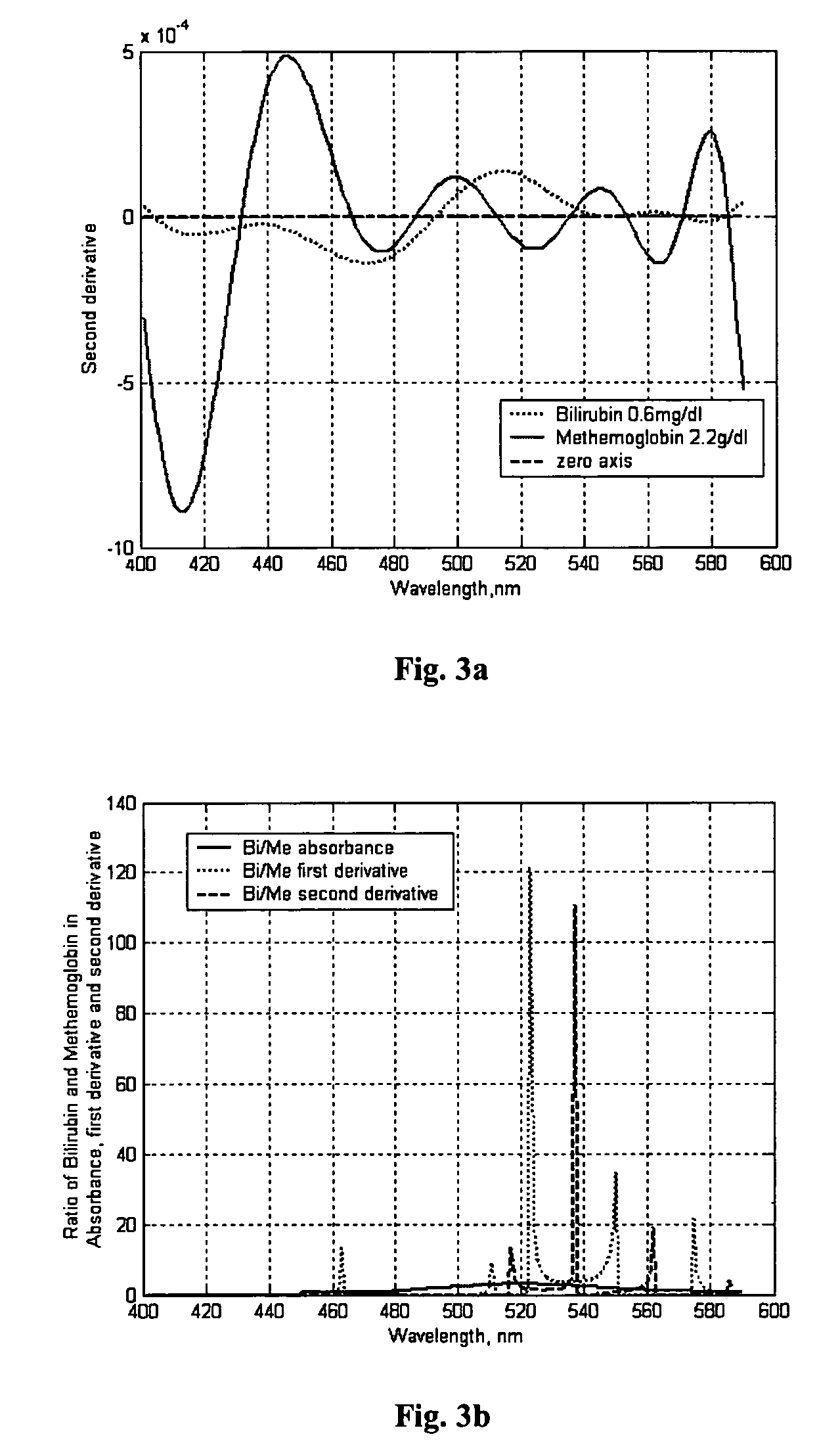
The present invention provides methods for assessing a condition in an individual by analyzing a cerebrospinal fluid sample from the individual, for example, by spectroscopy. Further, the invention provides methods for determining an amount of blood in the cerebrospinal fluid sample; methods for determining concentrations of analytes in the cerebrospinal fluid sample; methods for determining a length of time the blood has been in the cerebrospinal fluid sample; and methods for rapidly obtaining a differential diagnosis between conditions indicated by blood in the cerebral spinal fluid. Moreover, the present invention provides instruments capable of rapidly assessing a condition in and individual by point-of-care analysis of a cerebral fluid sample from an individual.
Owner:UNIVERSITY OF CINCINNATI
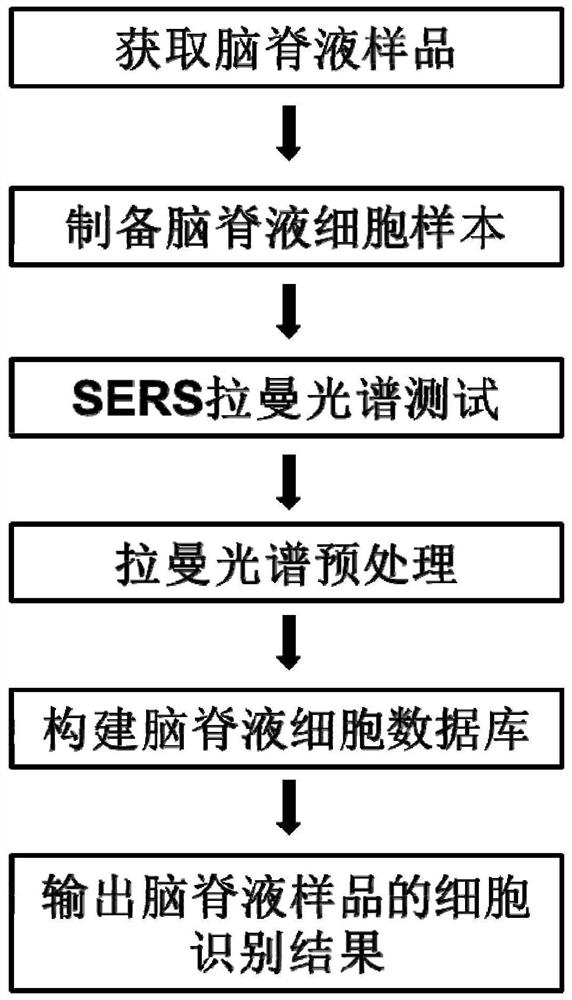
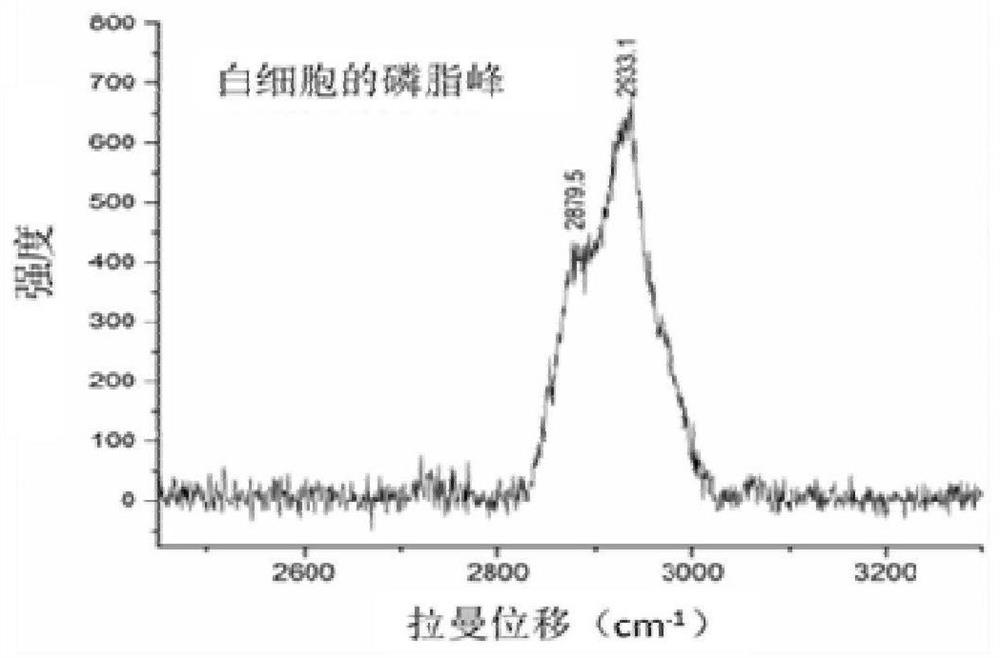
Cerebrospinal fluid cell detection method and system based on Raman scattering spectrum
ActiveCN111707656AReduce labor intensityFast wayRaman scatteringRaman scattering spectraPrincipal component analysis
The invention discloses a cerebrospinal fluid cell detection method and system based on a Raman scattering spectrum. The method comprises the following steps: 1, preparing a cerebrospinal fluid sample; 2, carrying out single-point test and imaging test on cerebrospinal fluid cells on the sample to obtain original Raman spectrum data; 3, processing the original Raman spectrum data obtained in the step 2 to obtain processed Raman spectrum data, wherein the processing comprises the steps of substrate removal preprocessing and normalization processing; and 4, carrying out data analysis on the Raman spectrum data processed in the step 3 by adopting a deep neural network and principal component analysis, and gathering the spectrums of cerebrospinal fluid cells of the same type together with datanodes of the same color to finish classification and detection. According to the method and the system, the detection and judgment accuracy can be improved, and meanwhile, the report issuing efficiency can be improved.
Owner:陕西未来健康科技有限公司
Anti-clogging ventricular catheter for cerebrospinal fluid drainage
A novel ventricular catheter designed to reduce CSF shunt obstruction is disclosed comprising a tip using a membrane without any opening and capable of filtering the CSF. When the CSF flows through the membrane, neither tissue (choroid plexus, blood cells, tumor cells, suctioned ependymal tissue) nor proteins can break through the membrane, making this ventricular catheter capable of preventing obstruction from tissue invasion but also preventing clogging from protein precipitation, coagulation or flocculation along the downstream shunt system.
Owner:LERS SURGICAL
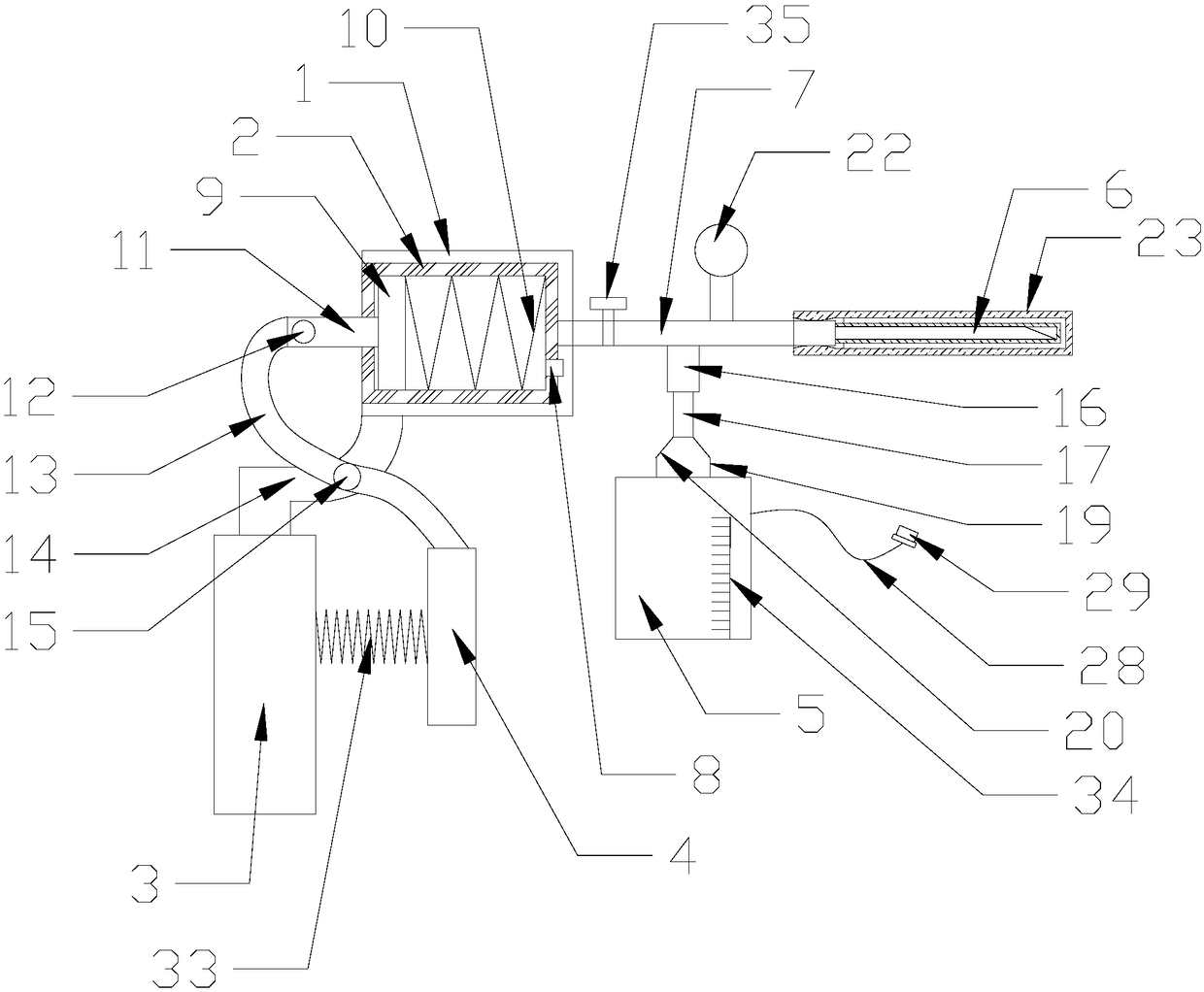
Neurological sampling device
InactiveCN109091170ASimple structureEasy to useSurgical needlesVaccination/ovulation diagnosticsCerebrospinal fluid specimenExhaust valve
A neurological sampling device comprises a U-shaped frame, a barrel, a handheld handle, a driving shank, a liquid storage bottle and a puncture needle. The barrel is arranged in the U-shaped frame, asuction tube and a one-way exhaust valve are arranged at one end of the barrel, a piston is arranged in the barrel, and a reciprocating spring is arranged between the piston and one end, with the suction tube, of the barrel. A transmission rod is arranged at the other end of the piston and extends out of the barrel to connect with a transmission connecting rod through a second rotating fastener. One side of the U-shaped frame is connected with the handheld handle through a linkage rod, the driving shank is arranged at the end of the transmission connecting rod, a connection tube is arranged atthe lower end of the suction tube, and the tail portion of the connection tube is connected with a flow guide tube through an anti-shedding mechanism. A conical end is hermetically connected with theflow guide tube, an annular cavity is formed between the outer wall of the flow guide tube and the interior of an outer sleeve, a pressure meter is arranged on the upper side of the suction tube, thepuncture needle is arranged at the front end of the suction tube, and the outer side of the suction tube is provided with a disposable sterilization cap. The neurological sampling device is simple instructure and convenient to use, pressure detection and sampling can be completed in one time, and cerebrospinal fluid sample contamination is avoided due to airtightness in a submission process.
Owner:杨彬
Method for detecting real-time fluorescence quota PCR of epidemic encephalitis B virus and kit
ActiveCN101942526AImprove accuracyGood precisionMicrobiological testing/measurementFluorescence/phosphorescenceForward primerNucleotide
The invention discloses a method for detecting real-time fluorescence quota PCR of epidemic encephalitis B virus and a kit. A primer and a TaqMan probe is designed in accordance with the conserved region of the epidemic encephalitis B virus E gene, which is used for quantitatively detecting nucleic acid copy number of the epidemic encephalitis B virus in samples. Particularly, the forward primer of the primer has a nucleotide sequence of SEQ ID NO: 1 in a sequence table, the reverse primer of the primer has a nucleotide sequence of SEQ ID NO: 2 in the sequence table, and TaqMan probe has a nucleotide sequence in SEQ ID NO: 3 in the sequence table. The invention has larger valuable significance in the field of controlling the quality of biological products related to the human and animal sources and inspecting and quarantining the imported and exported animals, ensures the quality of the related biological products and controls the spread of the epidemic encephalitis B and the medication safety of people. The detection method and the kit of the invention can be used for inspecting the epidemic encephalitis B virus on cerebrospinal fluid samples of the clinical patients, miniature pig brain tissue samples and the biological products, and have wide application prospect.
Owner:NAT INST FOR THE CONTROL OF PHARMA & BIOLOGICAL PROD
Methods and kits for the detection of cancer infiltration of the central nervous system
This invention relates to methods to detect the presence of cancer infiltration of the Central Nervous System (CNS) based on the detection of soluble proteins, preferably, in cerebrospinal fluid samples and vitreous fluid. The invention also relates to kits to perform the methods of the invention.
Owner:UNIV DE SALAMANCA
Gene chip for detection of cerebrospinal fluid pathogens
InactiveCN105063759AEasy to operateHigh detection specificityNucleotide librariesMicrobiological testing/measurementNucleotideCerebrospinal fluid sample
Belonging to the field of gene chips, the invention relates to a gene chip for detection of cerebrospinal fluid pathogens. The gene chip provided by the invention includes a substrate and probes, and the probes are fixed on the substrate. Specifically, the probes are nucleotide sequences as shown in SEQ ID NO.1-153, and the substrate is one of a glass slide, a silicon chip, a film and a high polymer material. The gene chip provided by the invention has the advantages of simple operation step, high detection specificity, good stability, short time and low cost, and can realize rapid, low cost, large flux and automatic detection of the species of pathogenic bacteria in cerebrospinal fluid samples of purulent meningitis patients, thus providing help for diagnosis of purulent meningitis.
Owner:GENERAL HOSPITAL OF NINGXIA MEDICAL UNIV
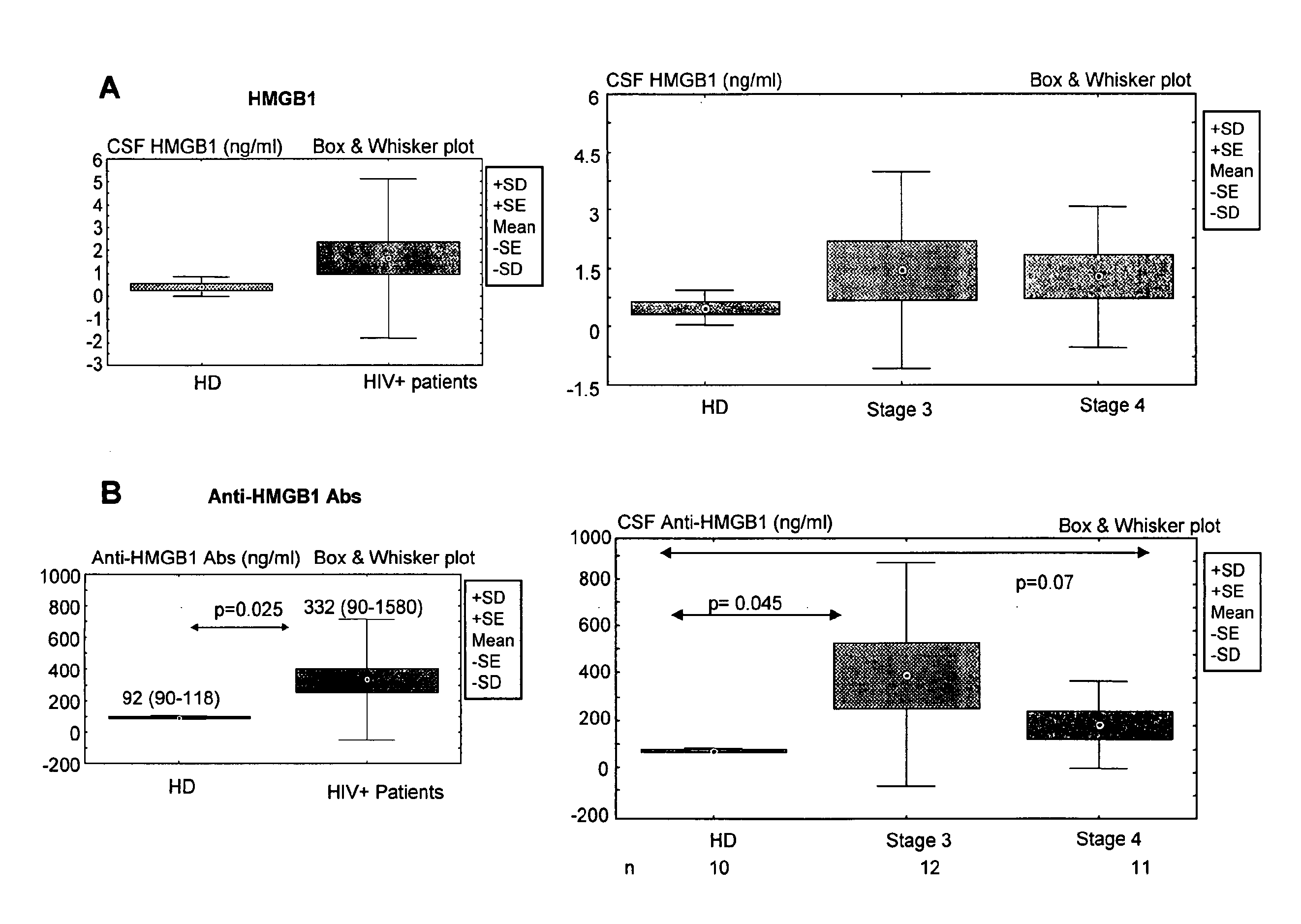
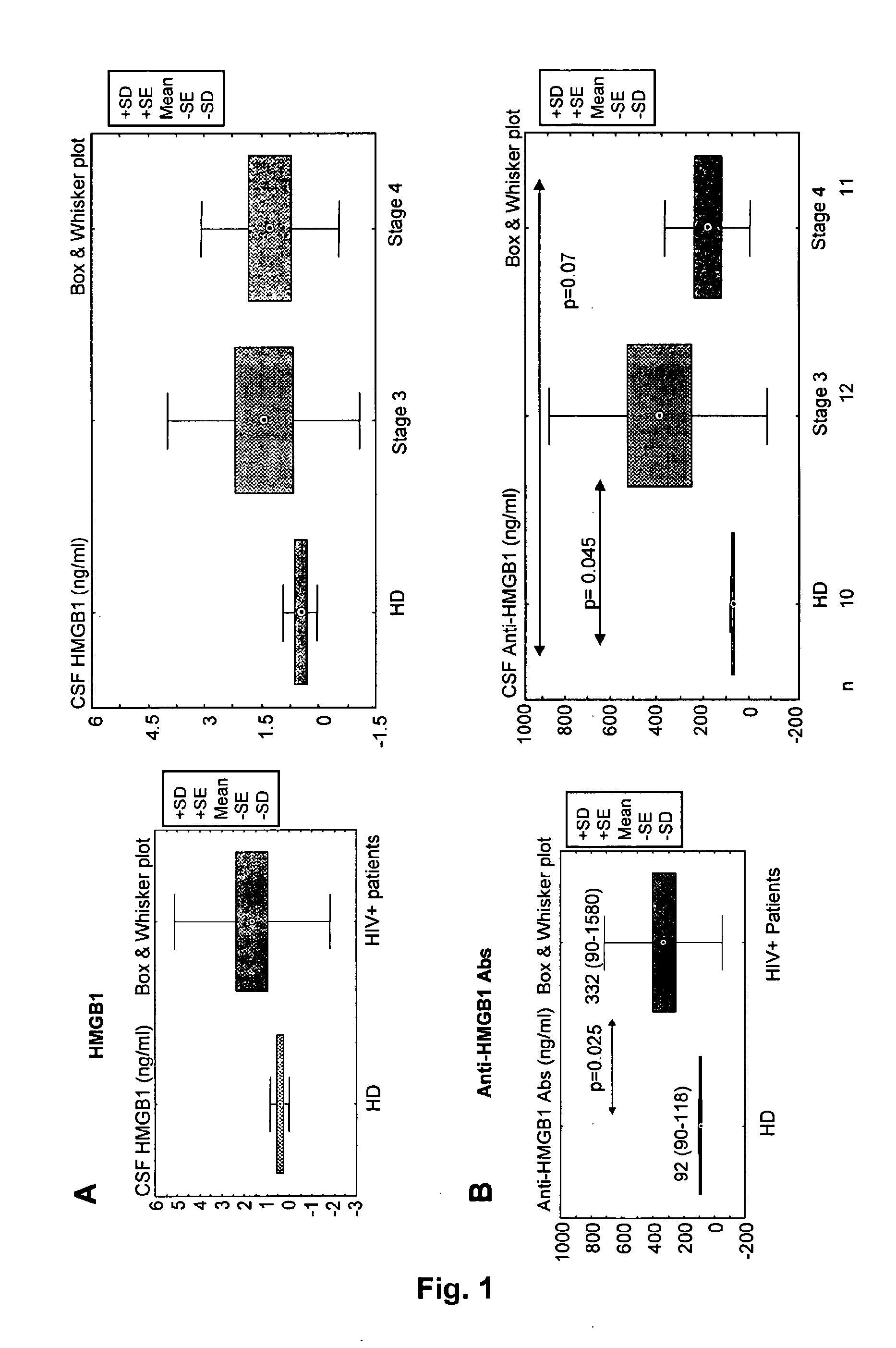
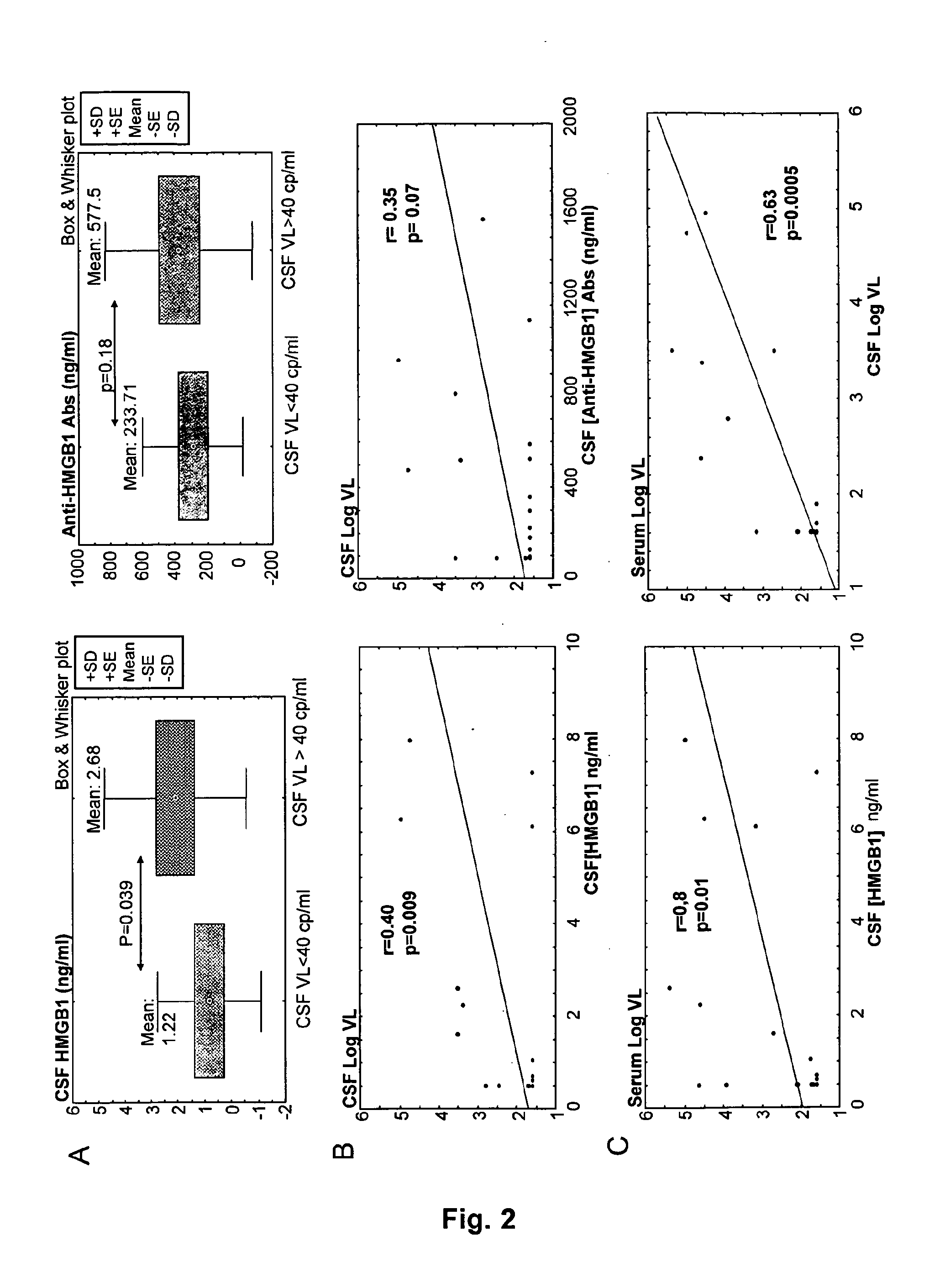
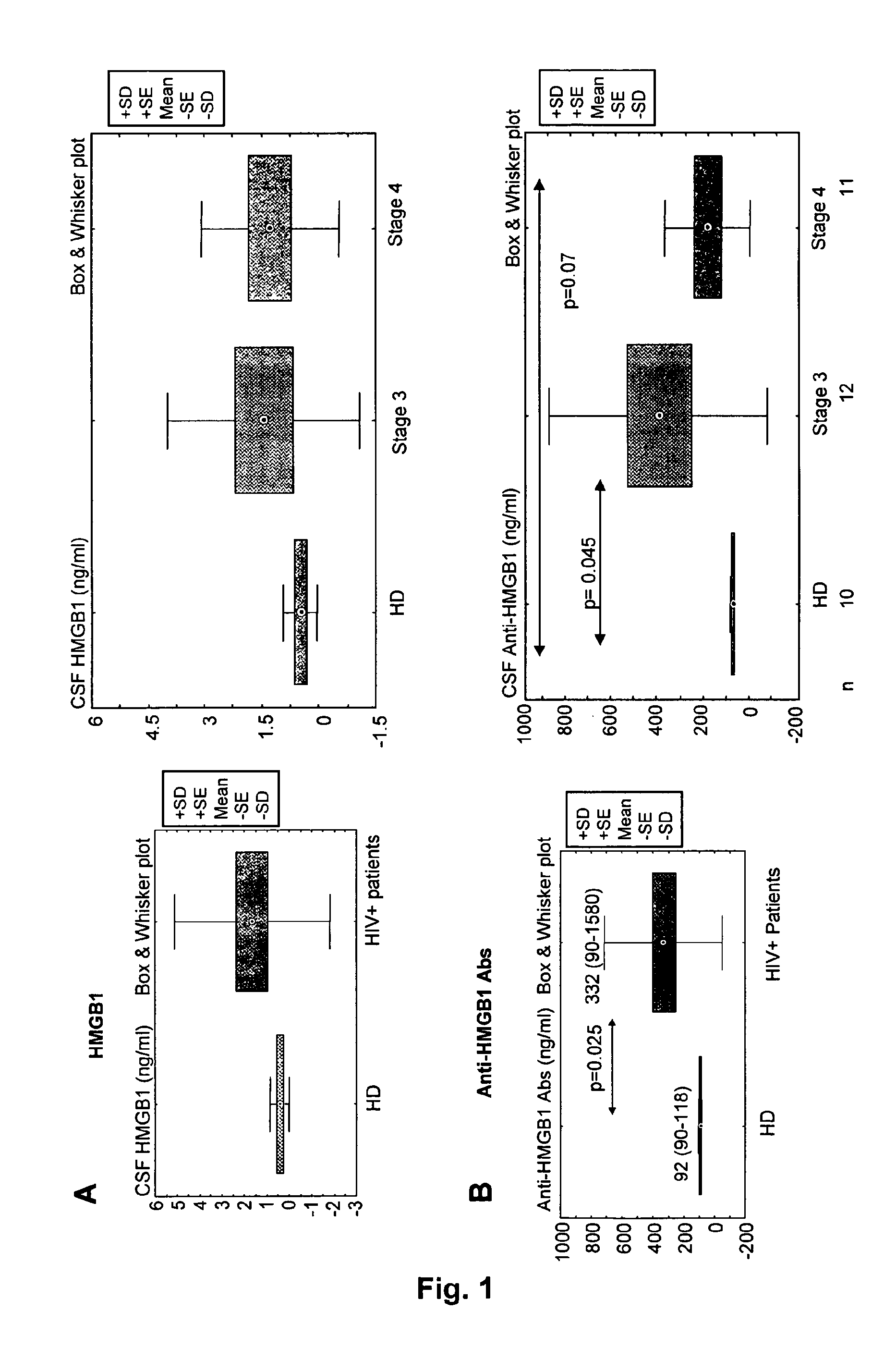
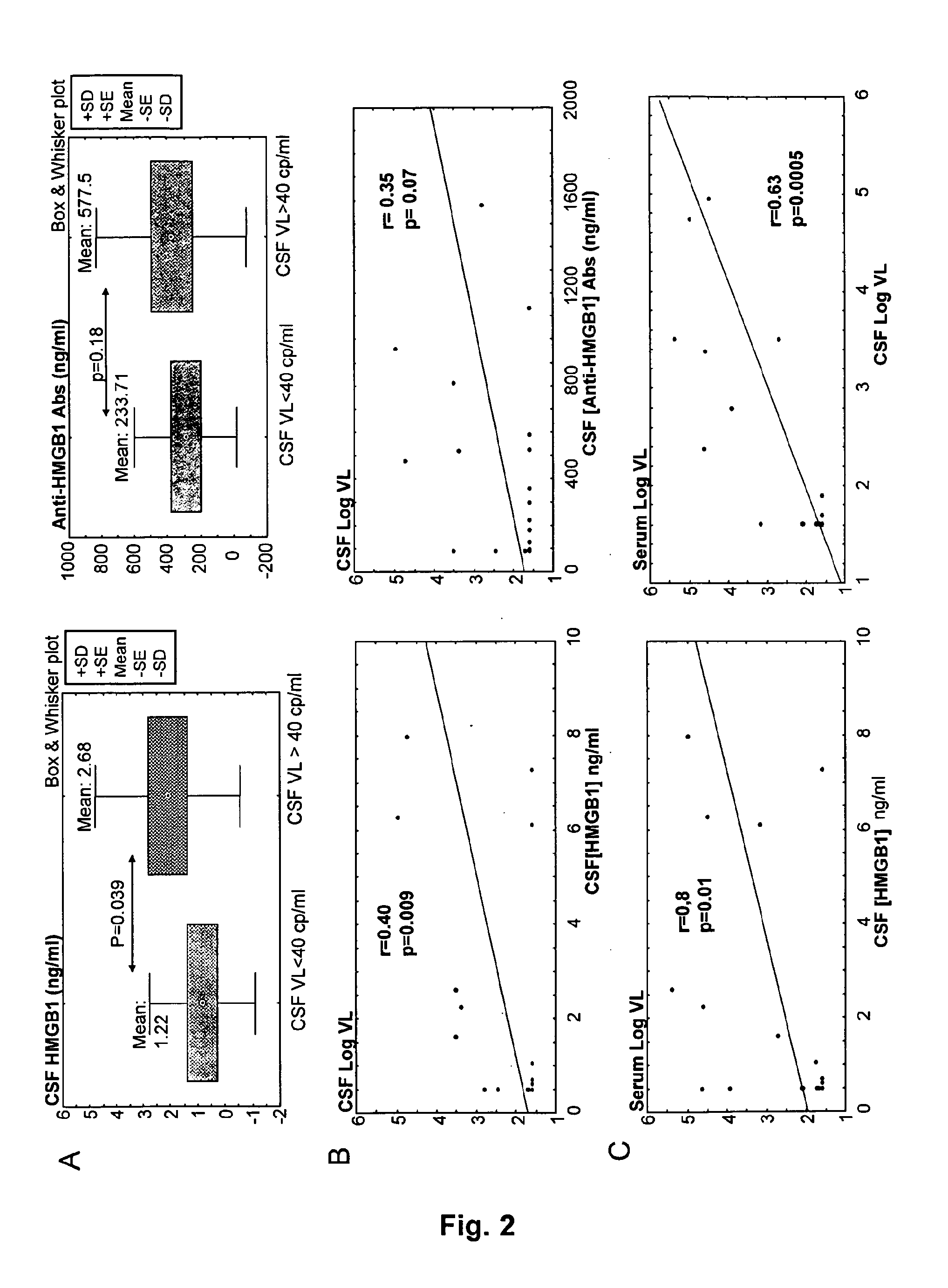
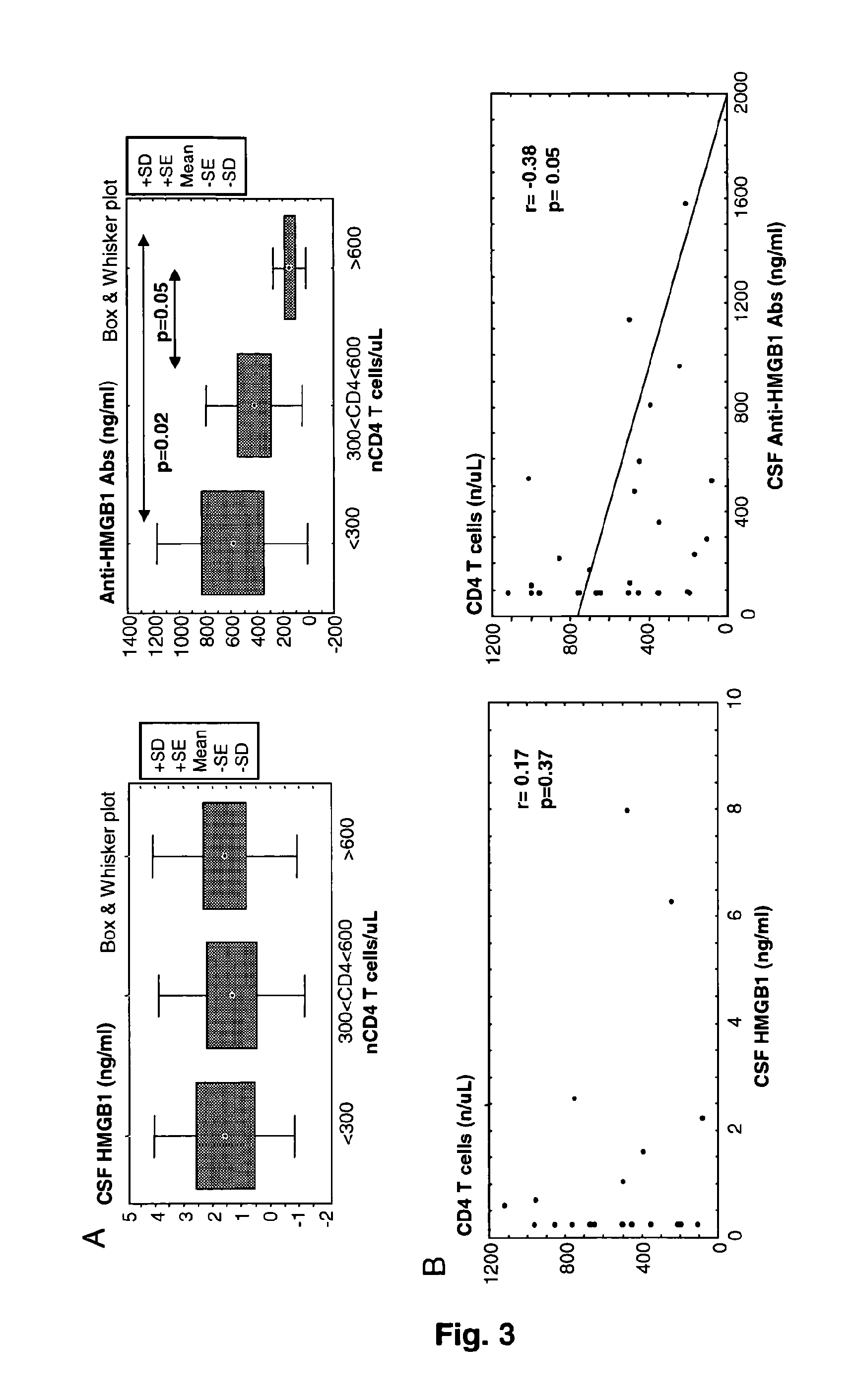
Hmgb1 and Anti-hmgb1 antibodies for the prognostic of neurological disorders
ActiveUS20130065221A1Microbiological testing/measurementVaccination/ovulation diagnosticsDiseaseNervous system
The invention relates to in vitro method for quantitating the antibodies specific for High mobility group box I (HMGB1) contained in a sample, in particular a serum sample or a cerebrospinal fluid sample obtained from a patient, and the use of this method in the prognostic and / or diagnosis of neurological disorders. These methods are in particular applicable to the monitoring of the human immunodeficiency virus (HIV) infection of a subject who is known to be infected with HIV and in the prognostic and / or diagnostic of the state of progression of Acquired immune deficiency syndrome (AIDS) or the state of progression toward AIDS, in particular the state of progression or the state of progression toward neurological disorders associated with AIDS. Finally, the invention is also about method to determine the immune deficiency or level of immune activation of a patient, in particular a HIV-infected patient.
Owner:INST PASTEUR
HMGB1 and anti-HMGB1 antibodies for the prognostic of neurological disorders
ActiveUS8728748B2Snake antigen ingredientsVaccination/ovulation diagnosticsNervous systemImmunodeficiency
The invention relates to in vitro method for quantitating the antibodies specific for High mobility group box I (HMGB1) contained in a sample, in particular a serum sample or a cerebrospinal fluid sample obtained from a patient, and the use of this method in the prognostic and / or diagnosis of neurological disorders. These methods are in particular applicable to the monitoring of the human immunodeficiency virus (HIV) infection of a subject who is known to be infected with HIV and in the prognostic and / or diagnostic of the state of progression of Acquired immune deficiency syndrome (AIDS) or the state of progression toward AIDS, in particular the state of progression or the state of progression toward neurological disorders associated with AIDS. Finally, the invention is also about method to determine the immune deficiency or level of immune activation of a patient, in particular a HIV-infected patient.
Owner:INST PASTEUR
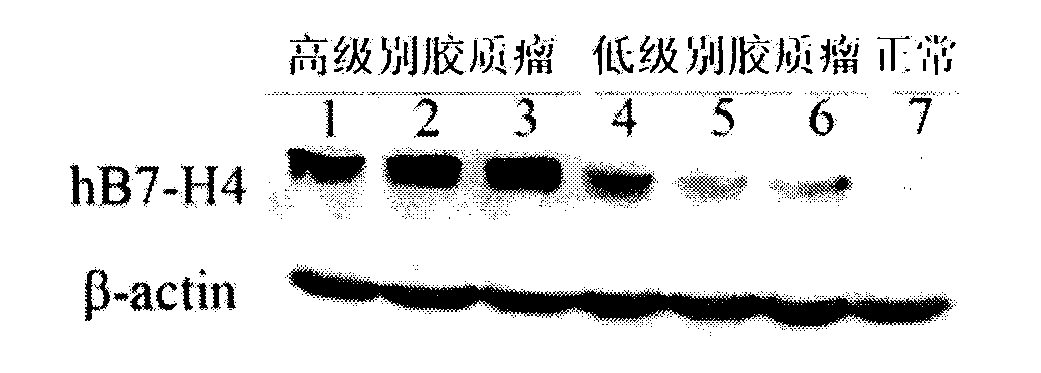
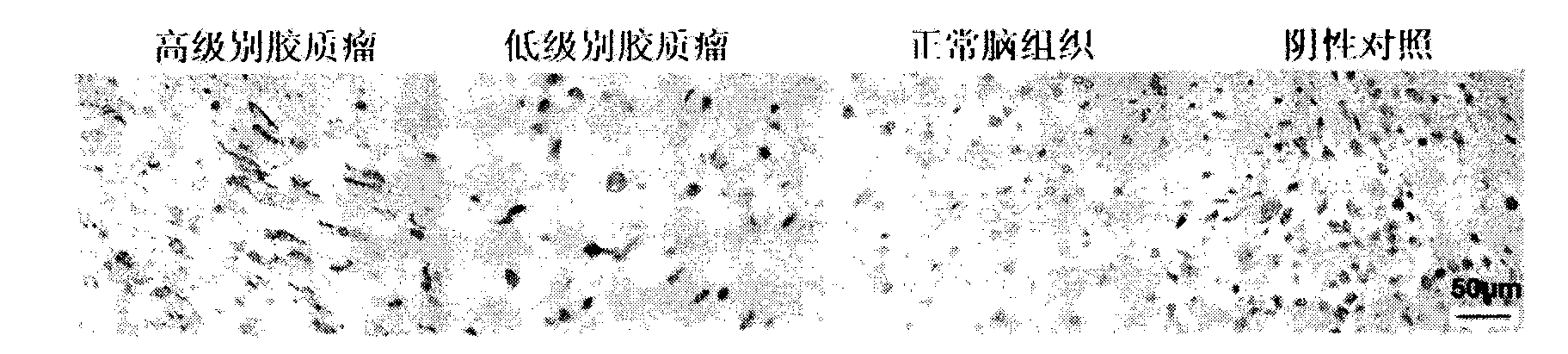
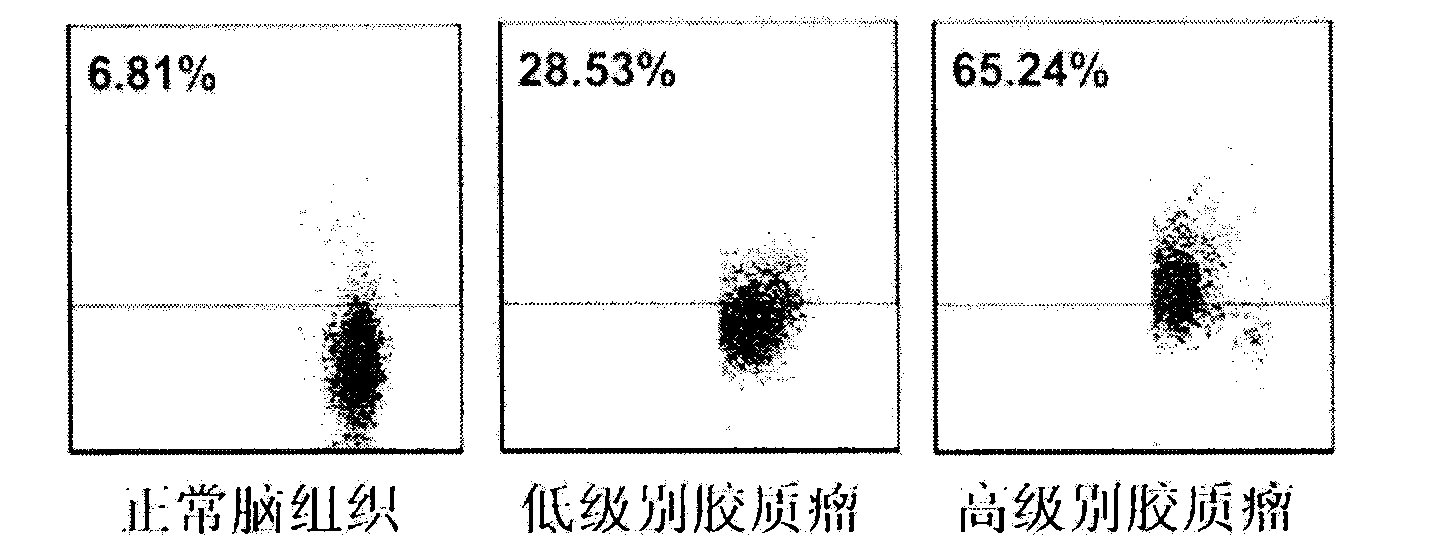
Kit for early diagnosis of glioma
ActiveCN104076151ATimely Auxiliary DiagnosisAccurate diagnosisDisease diagnosisBiological testingDiseasePeroxidase
The invention belongs to the technical field of biology, and relates to a kit for tumor diagnosis, in particular to a kit for early diagnosis of glioma. The kit comprises a mouse anti-human B7-H4 biotin labeling monoclonal antibody and streptavidin-peroxidase working liquid, a collected cerebrospinal fluid sample is detected, and corresponding diagnosis can be made by detecting B7-H4 molecules in the cerebrospinal fluid. The kit can be used for quantitatively detecting the B7-H4 protein expression quantity in the cerebrospinal fluid, so that the glioma infection risk can be predicted, people at high risk can be screened, the early diagnosis of glioma can be realized, and glioma and other intracranial space occupying diseases can be distinguished. The kit has the characteristics that the sensitivity is 70.8 percent, the specificity is 100 percent, the sensitivity for distinguishing low-grade and top-grade glioma is 87.5 percent, and the specificity for distinguishing low-grade and top-grade glioma is 75.0 percent. The kit is free of cross reaction with other cell factors, so that the powerful technical support is provided for the accurate diagnosis and prevention of glioma.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Pressure monitoring and releasing device for cerebrospinal fluid
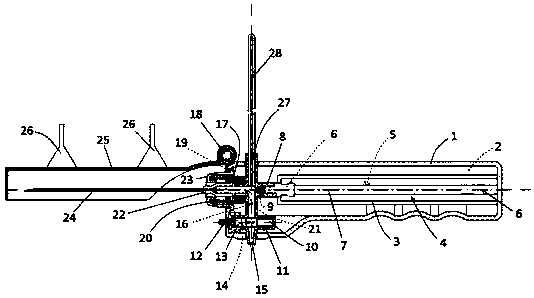
PendingCN108338782APrevent outflowSynchronization processSurgeryVaccination/ovulation diagnosticsVisibilityIntensive care medicine
The invention discloses a pressure monitoring and releasing device for cerebrospinal fluid. The pressure monitoring and releasing device is mainly applied to cerebrospinal fluid puncture needed in a clinical diagnosis and treatment process, for example, during lumbar puncture, pressure test of the cerebrospinal fluid is performed, a cerebrospinal fluid sample is retained for laboratory examination, and the like. The pressure monitoring and releasing device is characterized by replacing a traditional glass pressure testing tube in the lumbar puncture process; the required cerebrospinal fluid sample amount is retained by using a same device, pressure reading of the cerebrospinal fluid as well as visibility and quantization of the retained cerebrospinal fluid is realized, the operation flow is simplified, and puncture complication is avoided.
Owner:苏州诺来宁医疗科技有限公司
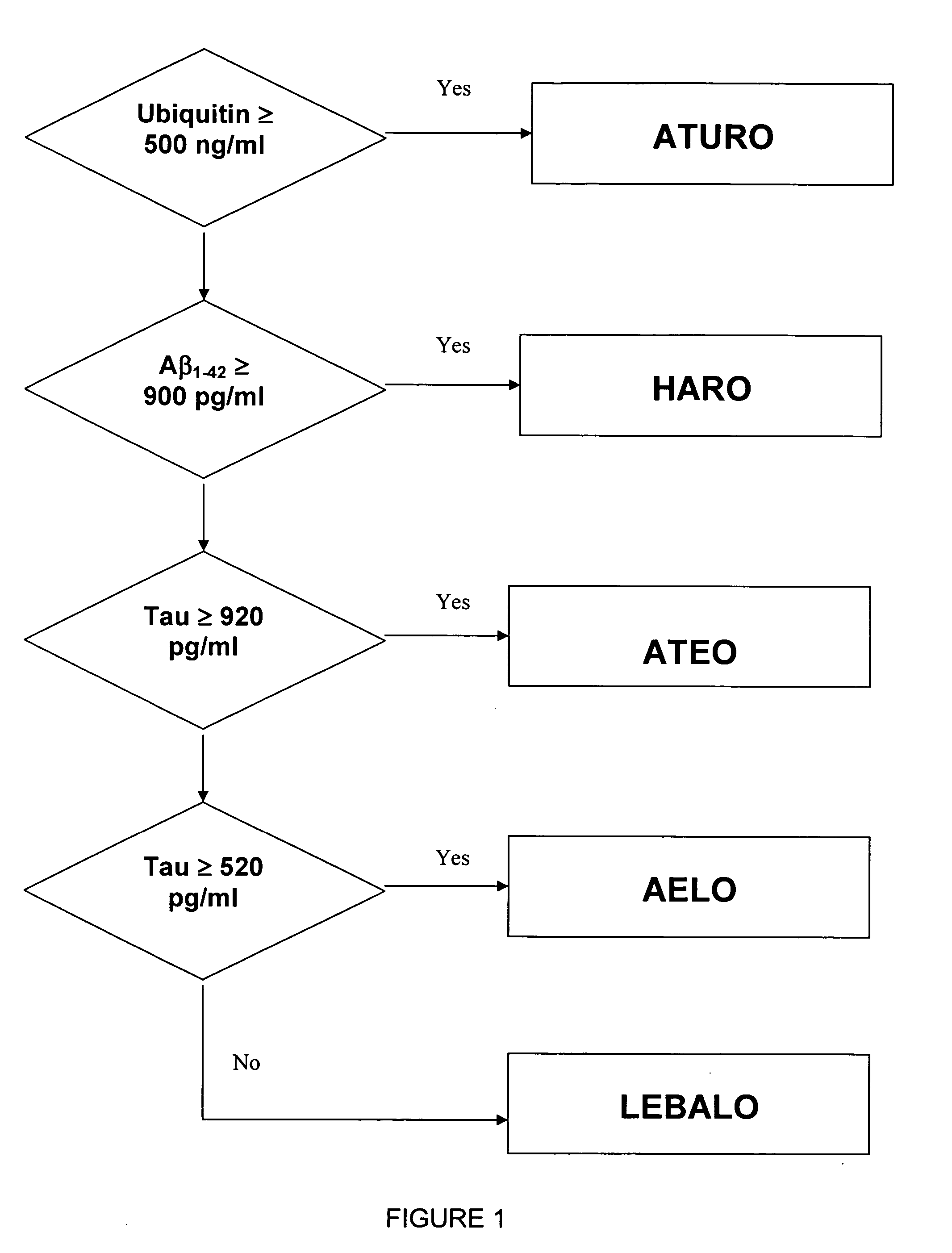
Method for differentiation of Alzheimer's disease into subgroups
A method for diagnosing distinct subgroups of Alzheimer's Disease, the method comprising the steps of obtaining a sample of cerebrospinal fluid and determining the level of ubiquitin, the level of Aβ1-42, and the level of tau present in the sample. Based on the levels of each composition in the cerebrospinal fluid, the sample can be assigned to distinct subgroups.
Owner:RES FOUND FOR MENTAL HYGIENE INC THE
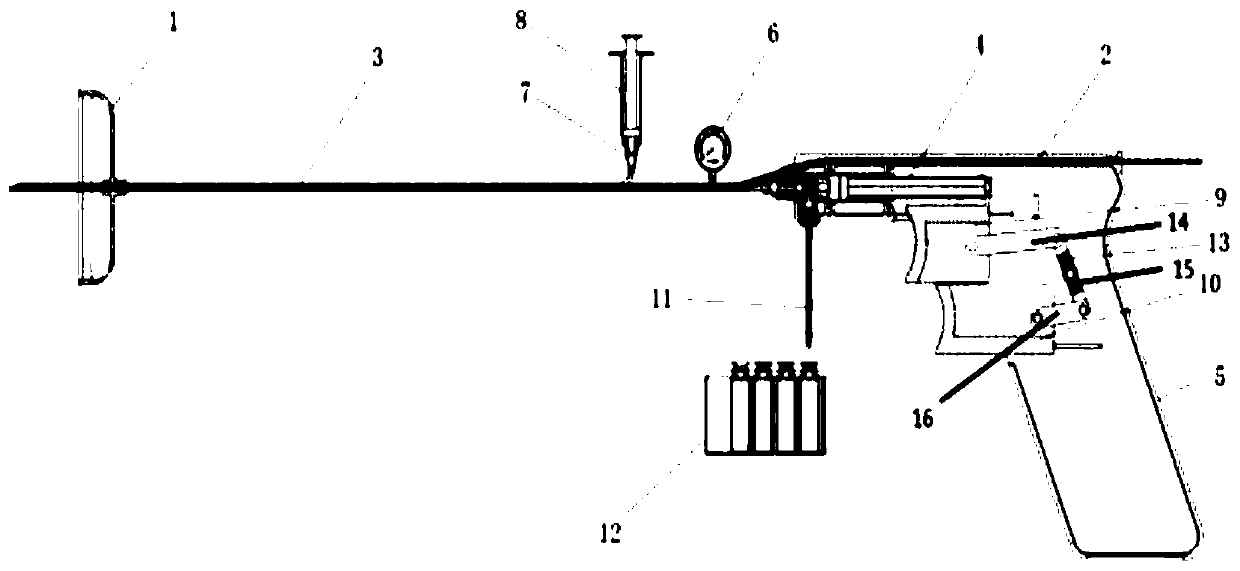
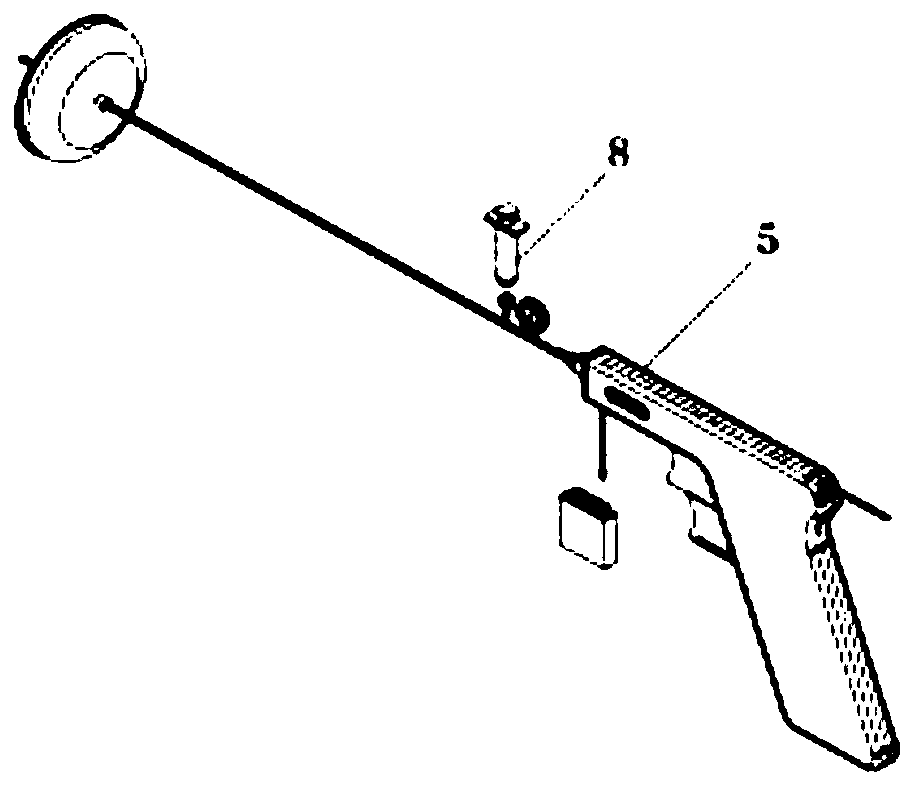
Pressure-measuring fluid-discharging injection gun for lumbar puncture
InactiveCN111110220AImprove accuracyImprove securityInfusion syringesSurgeryCerebrospinal fluid pressurePositive pressure
The invention discloses a pressure-measuring fluid-discharging injection gun for lumbar puncture. A cerebrospinal fluid collection trigger and a cerebrospinal fluid discharge trigger are arranged on an operation gun; the cerebrospinal fluid collection trigger is slidably hinged to the cerebrospinal fluid discharge trigger via a connection structure; a muzzle end of the operation gun is provided with a one-way valve injector; a push rod of the one-way valve injector is fixedly connected to the cerebrospinal fluid collection trigger; an inlet end of the one-way valve injector is provided with acollection one-way valve, and an outlet end of the one-way valve injector is provided with a discharge one-way valve; the inlet end is in airtight communication with a puncture needle; the cerebrospinal fluid collection trigger is used to form a negative pressure in the one-way valve injector; and the cerebrospinal fluid discharge trigger is used to form a positive pressure in the one-way valve injector through the connection structure. The pressure-measuring fluid-discharging injection gun provided by the invention achieves dynamic monitoring of a cerebrospinal fluid pressure, preservation ofa cerebrospinal fluid sample, and injection of a contrast agent and a drug at a time, and implements optimization of pressure measurement, fluid discharge and injection processes of the lumbar puncture.
Owner:HARBIN MEDICAL UNIVERSITY
Integrated safety lumbar puncture outfit
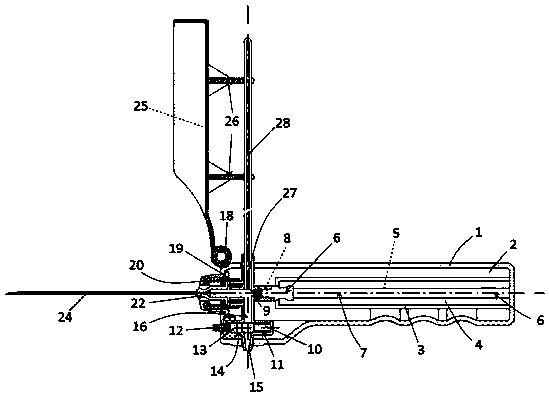
InactiveCN109984824ASurgical needlesVaccination/ovulation diagnosticsSubarachnoid spaceCerebrospinal fluid pressure
The invention discloses an integrated safety lumbar puncture outfit. The integrated safety lumbar puncture outfit is formed by a puncture unit, a cerebrospinal fluid circulating unit, a safety protection unit, and a machine housing. The integrated safety lumbar puncture outfit is mainly applied to needed conditions that a cerebrospinal fluid pressure test which needs to perform lumbar puncture isperformed in clinic diagnosis and treatment processes, a cerebrospinal fluid sample is reserved to perform laboratory examination, and intrathecal injection is performed through subarachnoid space andthe like. Compared with a traditional lumbar puncture needle, the integrated safety lumbar puncture outfit is capable of realizing the cerebrospinal fluid pressure test and the intrathecal injectionin the same device, reserving the cerebrospinal fluid sample according to the need, and controlling a release speed of cerebrospinal fluid, thereby avoiding a cerebral hernia risk caused by uncontrollable rapid cerebrospinal fluid release in a lumbar puncture process, protecting life safety of a lumbar puncture patient, and simpler and more healthful in operation.
Owner:迈德微创(天津)医疗器械有限责任公司
Novel preservation solution for microbial nucleic acid in cerebrospinal fluid
PendingCN111944877AInhibitory activityInhibitors can inhibit the activity ofMicrobiological testing/measurementLysisNuclease inhibitor
The invention discloses a novel preservation solution for microbial nucleic acid in cerebrospinal fluid. The fluid comprises the following components of a nuclease inhibitor, a buffer solution, a protein denaturation inhibitor, trisodium citrate, a bacteriostatic agent and a stabilizer, and the pH value of the novel preservation solution for the microbial nucleic acid in the cerebrospinal fluid is7-8. According to the novel preservation solution for the microbial nucleic acid in the cerebrospinal fluid, the activity of nuclease can be rapidly and effectively inhibited, the nucleic acid in a cerebrospinal fluid sample is prevented from being degraded, the content of the nucleic acid in the cerebrospinal fluid is guaranteed, and meanwhile, the cell lysis is avoided, the detection backgroundnoise is reduced, and the accuracy of cerebrospinal fluid detection is improved; and the preservation fluid is relatively simple in components and low in cost, and meanwhile, the stability of the free nucleic acid can be maintained at room temperature, and the fluid is extremely suitable for wide clinical application.
Owner:广州源古纪科技有限公司
Methods for diagnosing and assessing neurological diseases
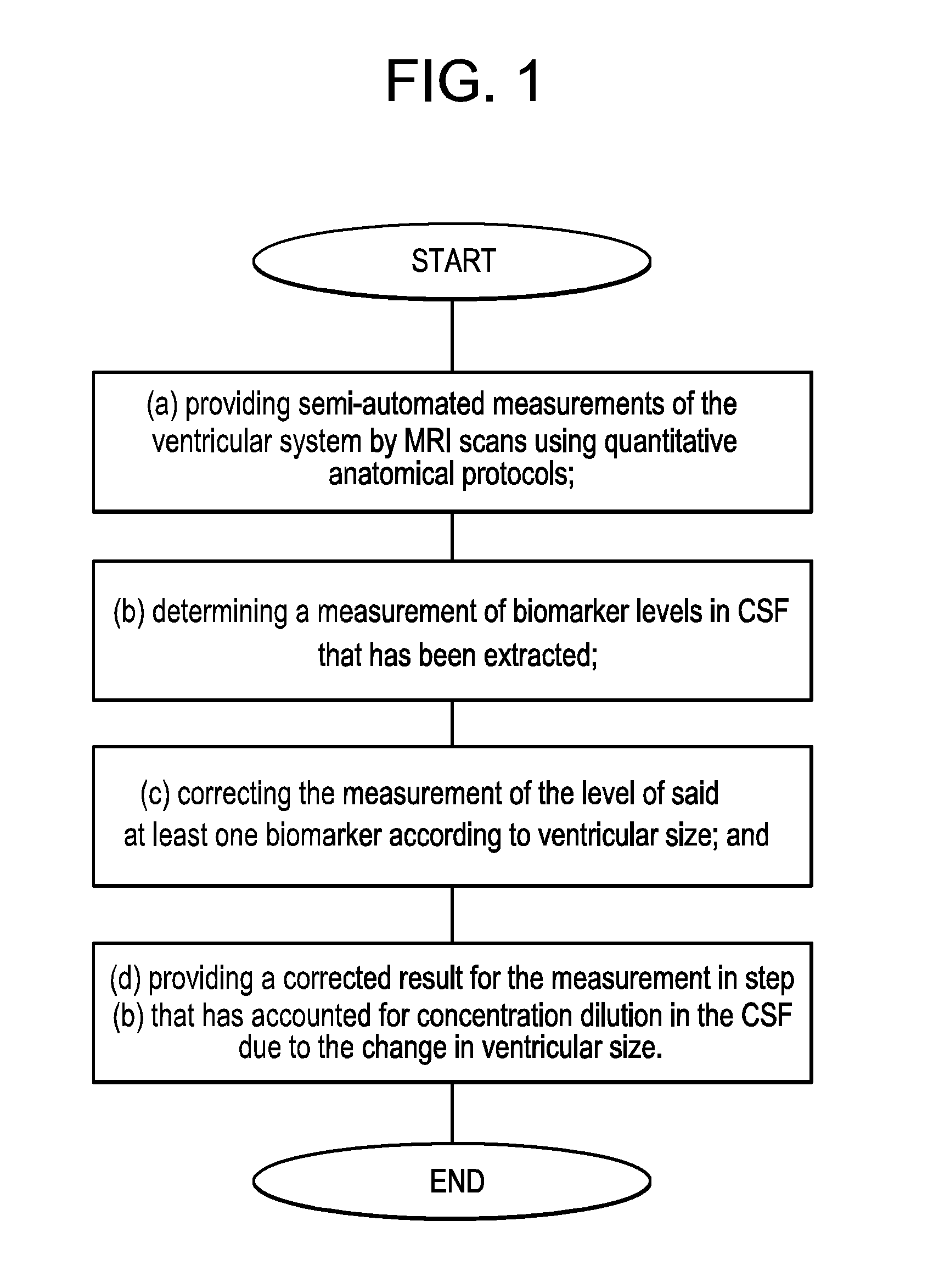
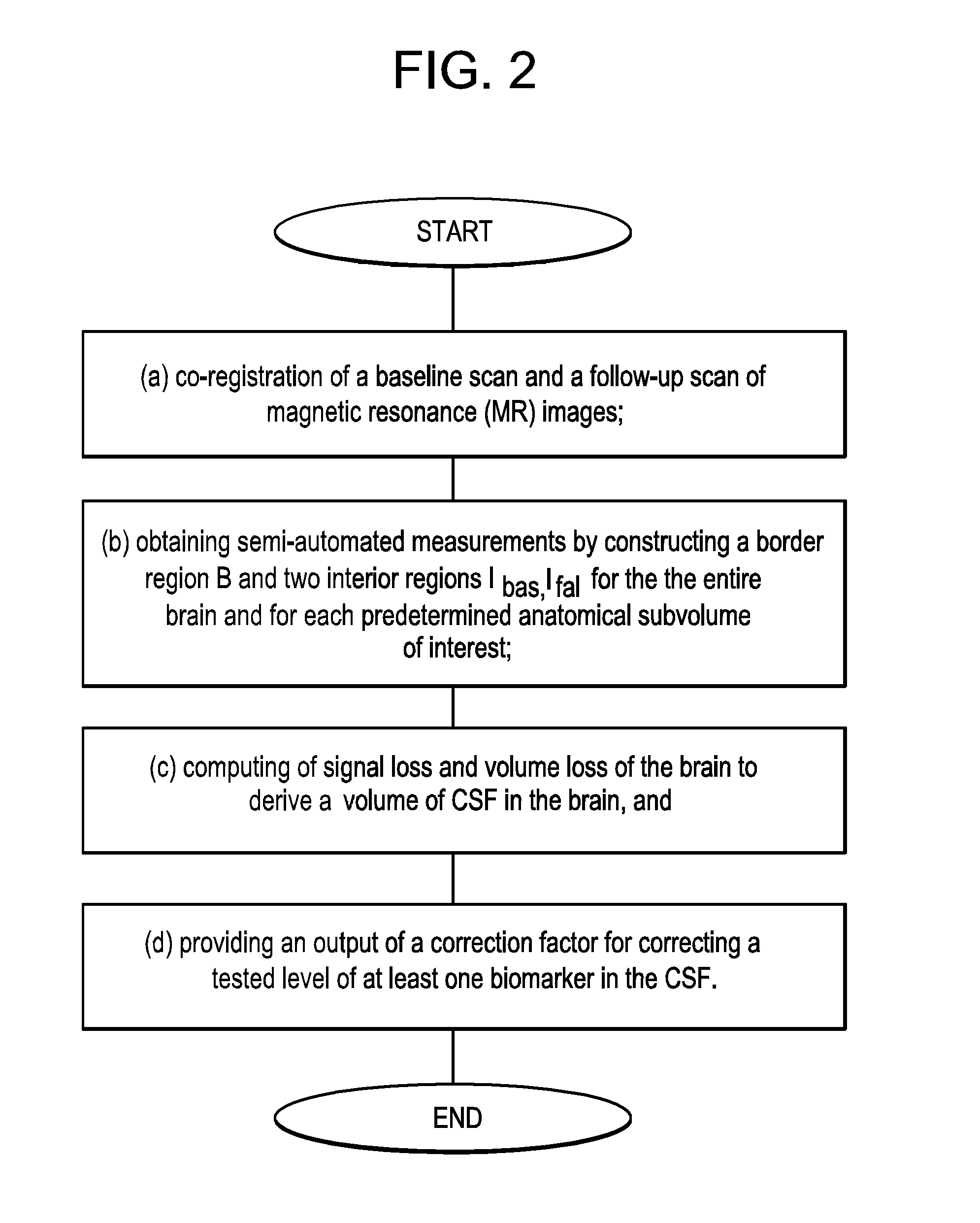
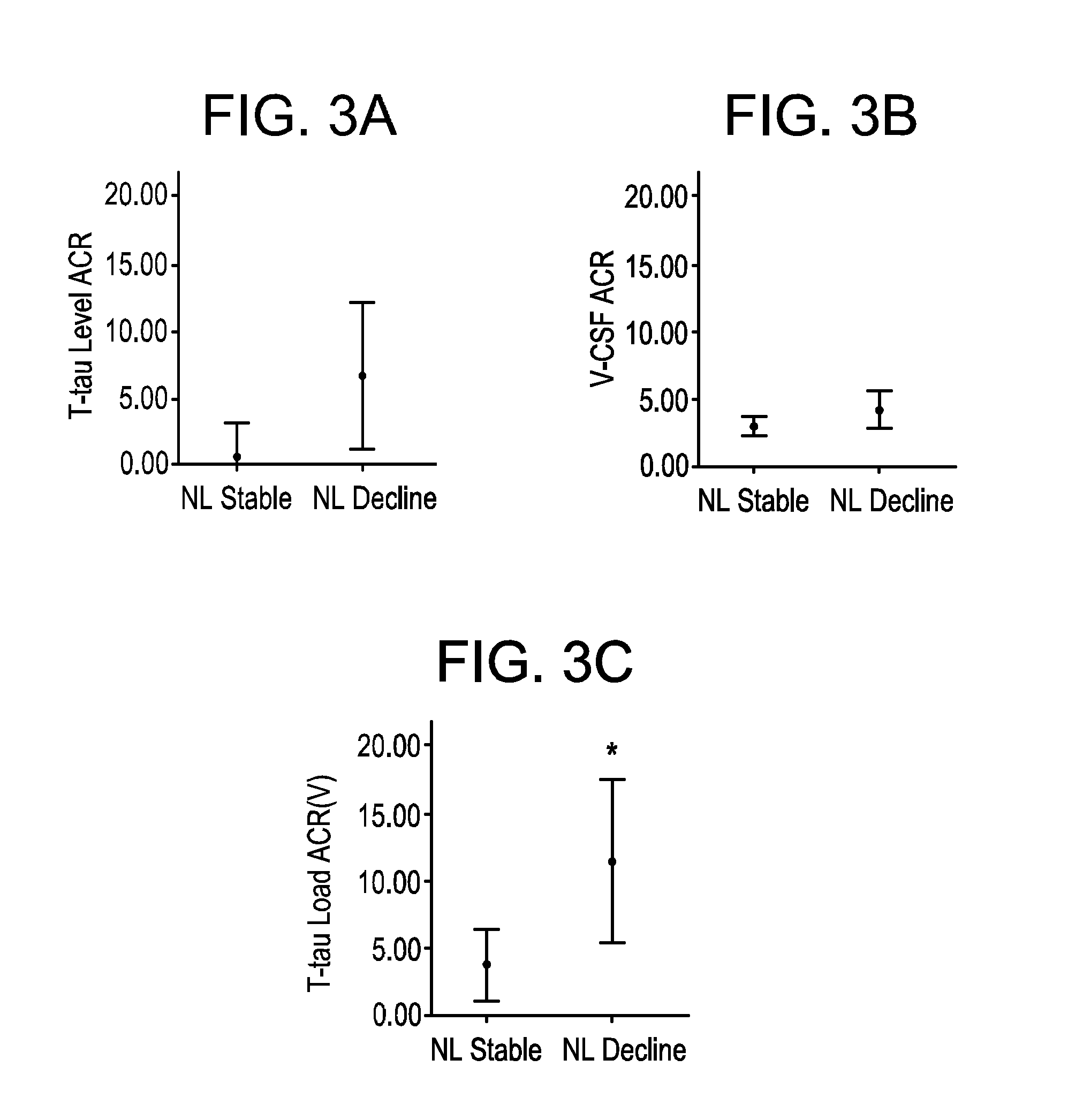
InactiveUS20160139150A1Rapid and sensitive screeningLower Level RequirementsMicrobiological testing/measurementDisease diagnosisMild cognitive impairment (MCI)Amyloid beta
The present invention provides methods for diagnosing a neurological disease in a subject, screening for or assessing the risk of developing a neurological disease in a subject, monitoring progression of a neurological disease in a subject, assessing efficacy of a therapy for a neurological disease in a subject, and identifying a subject suffering from a neurological disease that may be successfully treated by an agent that affects levels of a biomarker such as a tau protein or an amyloid beta. The methods generally feature (a) obtaining a cerebrospinal fluid (CSF) sample from a subject; (b) providing a cerebrospinal fluid (CSF) correction factor for CSF obtained from a subject for at least one biomarker; and (c) determining whether the biomarker is present in elevated amounts or concentrations in the cerebrospinal fluid (CSF) sample. The neurological disease may be, for instance, impaired cognition or dementia such as Alzheimer's Disease (AD) or Mild Cognitive Impairment (MCI). The biomarker may be a tau protein such as P-tau231 or an amyloid beta (Aβ) such as Aβ42 or Aβ40.
Owner:NEW YORK UNIV
Multiplexed biomarkers for monitoring the Alzheimer's disease state of a subject
The present invention relates to a method for diagnosing a subject's Alzheimer's disease state. The method involves providing a database containing information relating to protein expression levels associated and not associated with Alzheimer's disease. The database includes information relating to at least a majority of the following proteins: albumin, alpha-1-antitrypsin, apolipoprotin E, apolipoprotein J, complement component 3, contactin, fibrin beta, Ig heavy chain, Ig light chain, neuronal pentraxin receptor, plasminogen, proSAAS, retinol-binding protein, transthyretin, and vitamin D binding protein. Information relating to proteins found in one or more cerebrospinal fluid samples from a subject is also provided and a database is used to analyze the information from the subject to diagnose the subject's Alzheimer's disease state. Also disclosed is a computer readable medium and a system, both useful in carrying out the present invention.
Owner:CORNELL RES FOUNDATION INC
Method for diagnosis
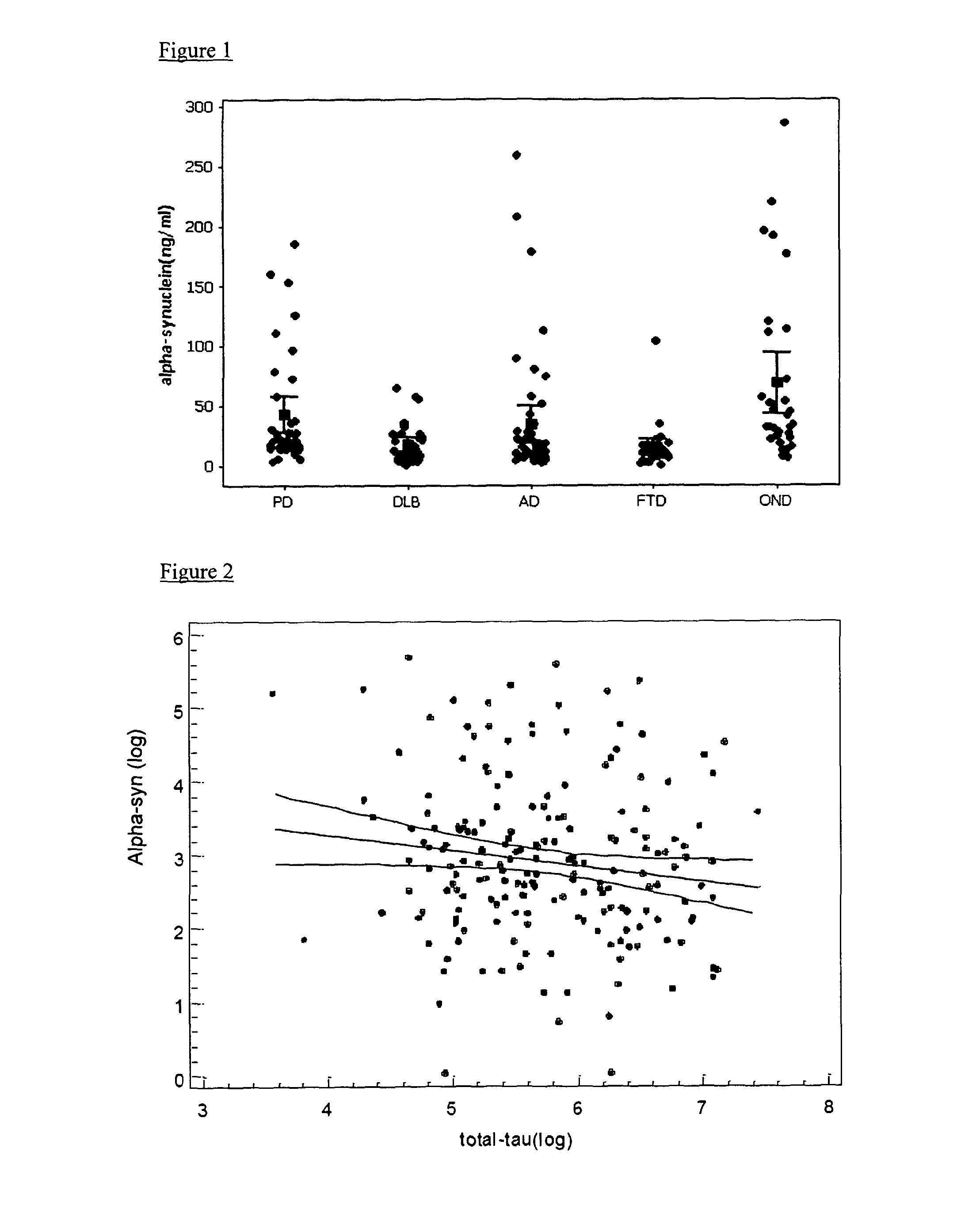
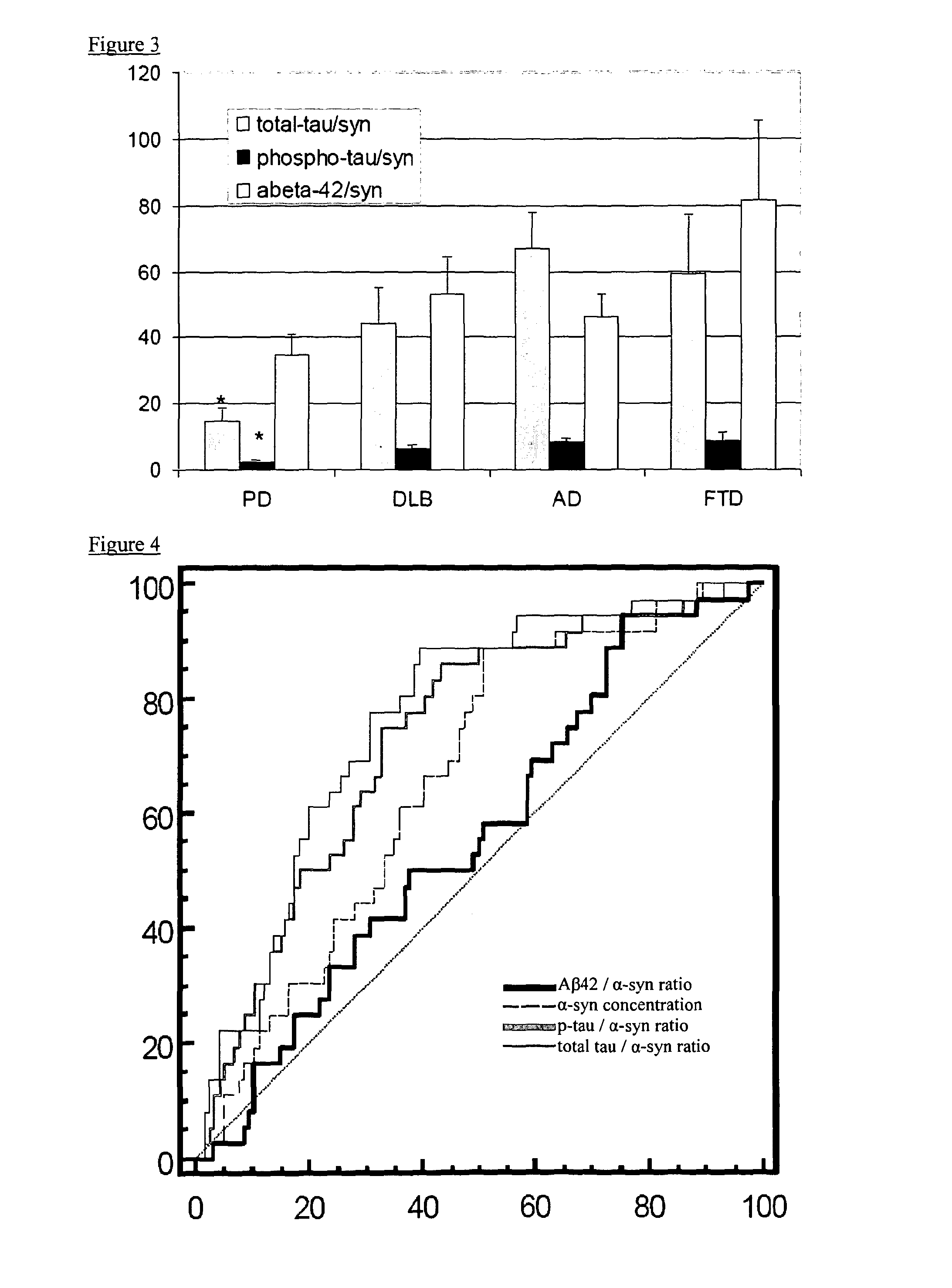
The present invention relates to method of identifying whether or not an individual has Parkinson's disease (PD). In particular, the invention relates to a method for identifying whether or not an individual has PD as opposed to another neurodegenerative disease. The method of the invention comprises measuring the concentration of α-synuclein (α-syn) and the concentration of unphosphorylated tau (tau) and / or phosphorylated tau (p-tau) in a cerebrospinal fluid sample taken from an individual. The method also comprises calculating the ratio of the concentration of tau and / or p-tau to the concentration of α-syn, and thereby determining whether or not the individual has PD.
Owner:UNITED ARAB EMIRATES UNIVERSITY +1
Molecular markers for auxiliary diagnosis of viral meningitis as well as application and kit of molecular markers
ActiveCN112779329AVarious clinical manifestationsImprove early diagnosis rateMicrobiological testing/measurementDNA/RNA fragmentationTreatment effectGene Microarray
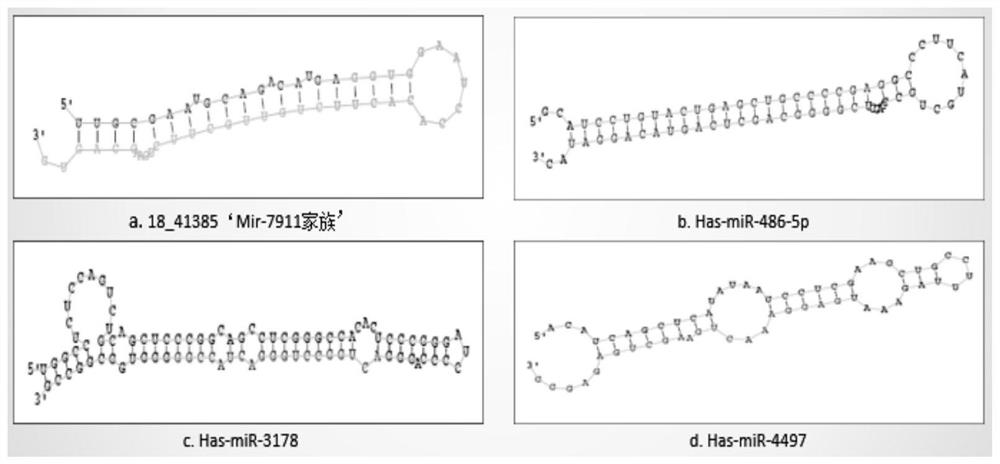
The invention discloses molecular markers for auxiliary clinical diagnosis of viral meningitis as well as application and a kit of the molecular markers. Firstly, systematic gene chip analysis is carried out on two groups of cerebrospinal fluid samples of viral meningitis and normal control by a miSeq high-throughput sequencing technology, differential miRNA expression is screened, the screened differential expression miRNA is further verified one by one by an RT-qPCR technology, and finally, it is found that miR-RNA 18_41385, hsa-miR-486-5p, hsa-miR-4497 and the hsa-miR-3178 can be used as specific markers for early auxiliary clinical diagnosis of the viral meningitis. The markers can also be used for differential diagnosis of other meningitis. The kit has the advantages of being good in stability, simple and convenient in detection method, high in sensitivity, specific and the like, achieves effective supplementation of an existing clinical viral meningitis diagnosis method and rapid definite diagnosis of viral meningitis patients, and is of great significance in reducing the death rate of the viral meningitis and improving the treatment effect and prognosis.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com