A kind of lacidipine solid dispersion and preparation method thereof
A technology of solid dispersion and lacidipine, applied in the field of medicine, can solve problems such as slow dissolution rate, and achieve the effects of improved dissolution rate, improved bioavailability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
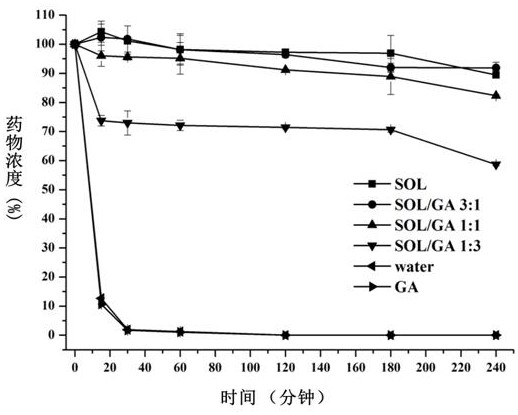
[0044] Embodiment 1 crystal nucleation inhibitor and dissolution accelerator ratio are on the influence of drug supersaturation (attachment figure 1 )
[0045] Choose Soluplus as the crystal nucleus inhibitor, and GA as the dissolution accelerator.
[0046] The effect of the ratio of crystal nucleus inhibitor / dissolution accelerator on the supersaturation of the drug was investigated by solvent change method. The operation method was as follows: the ratio of lacidipine / carrier material was fixed at 1:5, and a certain amount of GA and Soluplus were weighed respectively. , Dissolved in 500mL PBS6.8 buffer solution. In addition, weigh 10 mg of lacidipine and add 1 mL of absolute ethanol to make a clear solution. With reference to the 2015 edition Chinese Pharmacopoeia Dissolution Determination Method (Appendix X C second method), the above-mentioned PBS buffer solution containing GA and Soluplus is used as the medium, and the rotating speed is 50 revolutions per minute, and the...
Embodiment 2
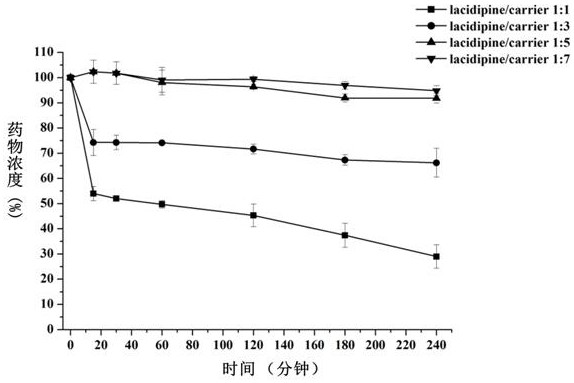
[0048] The impact of embodiment 2 carrier material proportions on the degree of supersaturation of medicine (attachment figure 2 )
[0049] Soluplus, GA was selected as the carrier material, and the effect of the ratio of drug / carrier material on the supersaturation of lacidipine was investigated by solvent change method.
[0050] The specific operation method is as follows: Weigh 10 mg of lacidipine, add 1 mL of absolute ethanol to make a clear solution. In addition, the ratio of Soluplus / GA was fixed at 3:1, and 500 mL of PBS6.8 buffer solutions containing different amounts of carrier material were prepared respectively, so that the ratio of lacidipine / carrier material was 1:1-1:7. With reference to the 2015 edition Chinese Pharmacopoeia Dissolution Determination Method (appendix X C second method), the above-mentioned PBS buffer solution containing different amounts of carrier materials is adopted as the medium, and the rotating speed is 50 revolutions per minute, and the...
Embodiment 3
[0052] The preparation of embodiment 3 lacidipine solid dispersion
[0053] Adopt spray-drying method to prepare lacidipine solid dispersion, typical preparation process is as follows:
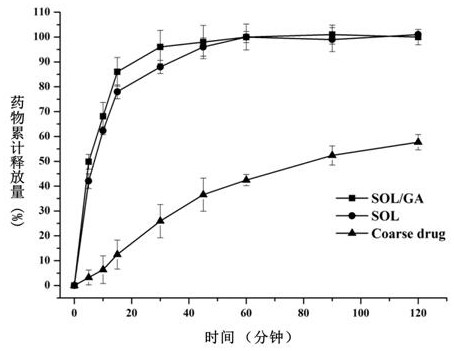
[0054] Preparation of ternary solid dispersion: Dissolve 750mg Soluplus and 250mg GA in 500mL distilled water to form solution A, and dissolve 200mg lacidipine in 50mL absolute ethanol to form solution B, add solution B to solution A, and stir until Form a clear solution, adopt spray drying method (Buchi 290mini spray drier, Switzerland) at 100 ℃ inlet temperature, 600m 3 Under the conditions of air flow rate / h and 2mL / min liquid supply speed, carry out spray-drying, make lacidipine ternary solid dispersion.
[0055] Preparation of binary solid dispersion (control group): 1000mg Soluplus was dissolved in 500mL distilled water to form solution A, 200mg lacidipine was dissolved in 50mL absolute ethanol to form solution B, solution B was added to solution A, and stirred until Clear solution, us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com