Mesosulide film-coated tablet and preparation method thereof
A technology of film-coated tablets and susulide, applied in the field of mesosulide excipients, mesosulide preparations and its preparation, to achieve good taste, good patient compliance, and convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment a1
[0099] Example a1: Research on the prescription of mesosulide film-coated tablets
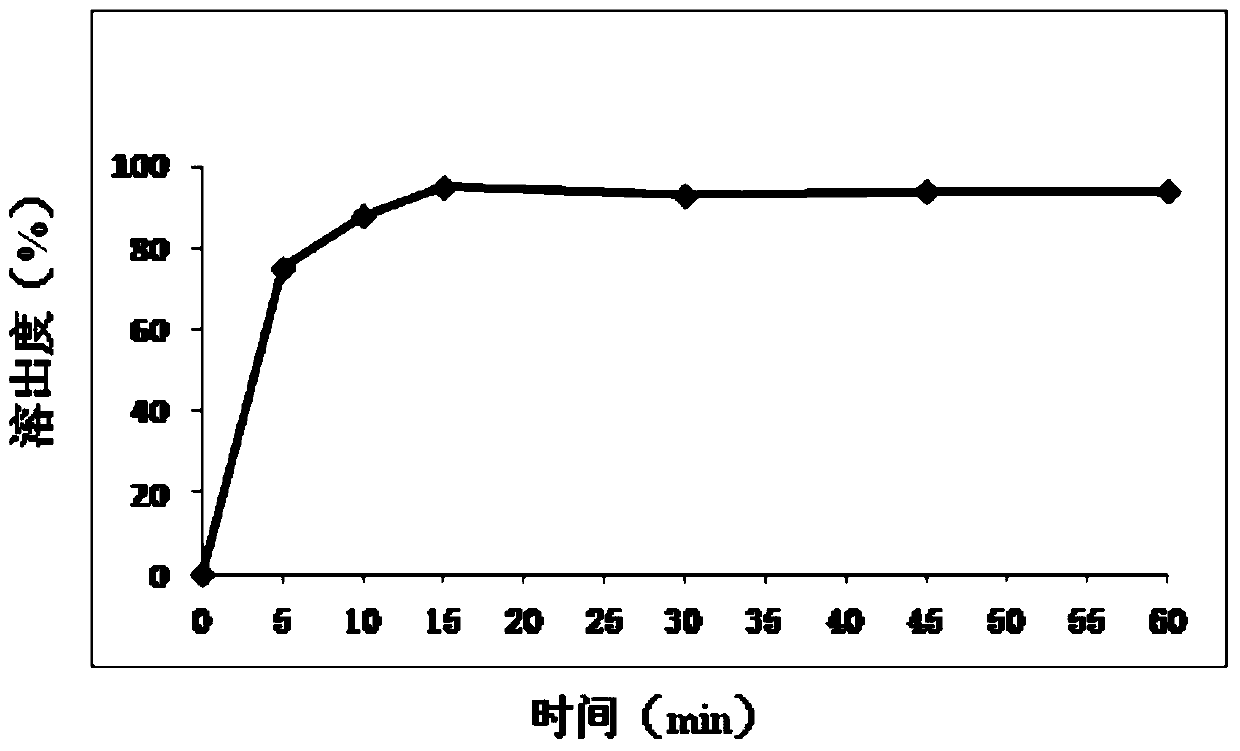
[0100] Since the main drug mesosulide is almost insoluble in water and the amount of the main drug is relatively large, the difficulty in the prescription process is to improve the dissolution rate of this product. In this example, the prescription of mesosulide film-coated tablets with a specification of 100 mg / tablet was studied. Specifically, due to the poor water solubility and fluidity of the raw material mesosulide, it was considered to add water-soluble excipients And disintegrants to promote dissolution, designed 9 prescriptions, see Table a1.
[0101] Table a1 Different formulation designs
[0102]
[0103]
[0104] Note: In the preparation of adhesives, hypromellose needs to be prepared with purified water to make 4% hypromellose aqueous solution (g / g), and povidone K30 needs to be added with purified water to make 8% polyvinyl alcohol. Aqueous solution of vitamin K30 (g / g), "...
Embodiment a2
[0123] Example a2: Mesosulide film-coated tablet prescription and process optimization of 100mg specification
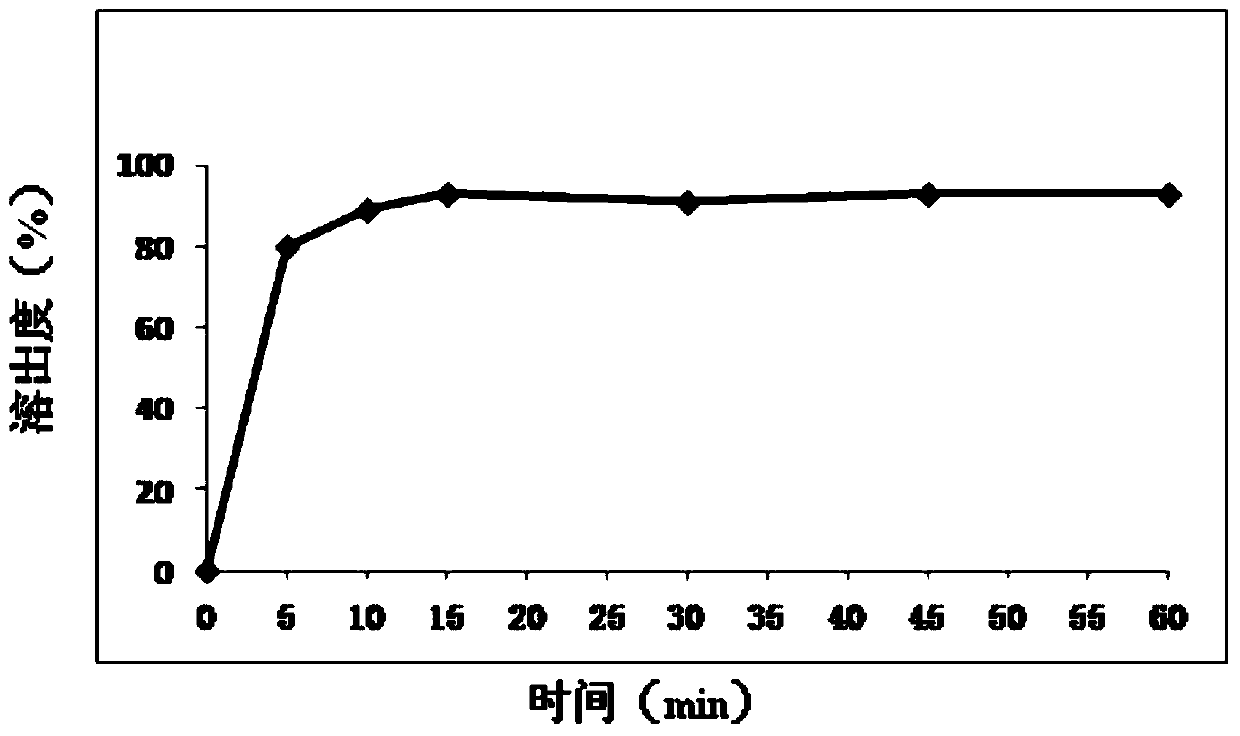
[0124] In this study, according to the dosage form requirements of the Chinese Pharmacopoeia 2010 edition, referring to the "Basic Technical Guidelines for the Research of Chemical Pharmaceutical Preparations" promulgated by CDE, combined with the characteristics of film-coated tablets, the properties, hardness, friability, disintegration time, Dissolution rate and so on were used as the investigation index, and the types and dosages of excipients used in the prescription of this product were further screened.
[0125] (1) Screening of fillers
[0126] Adjust the ratio and dosage of microcrystalline cellulose, and investigate the effect of different dosages of diluent (in this article, "filler" and "diluent" can be used interchangeably) on the tablet formability and dissolution rate. The specific prescription and results are as follows :
[0127]In this study, micr...
Embodiment a3
[0164] Example a3: Further optimization experiment of the preparation process of mesosulide film-coated tablets
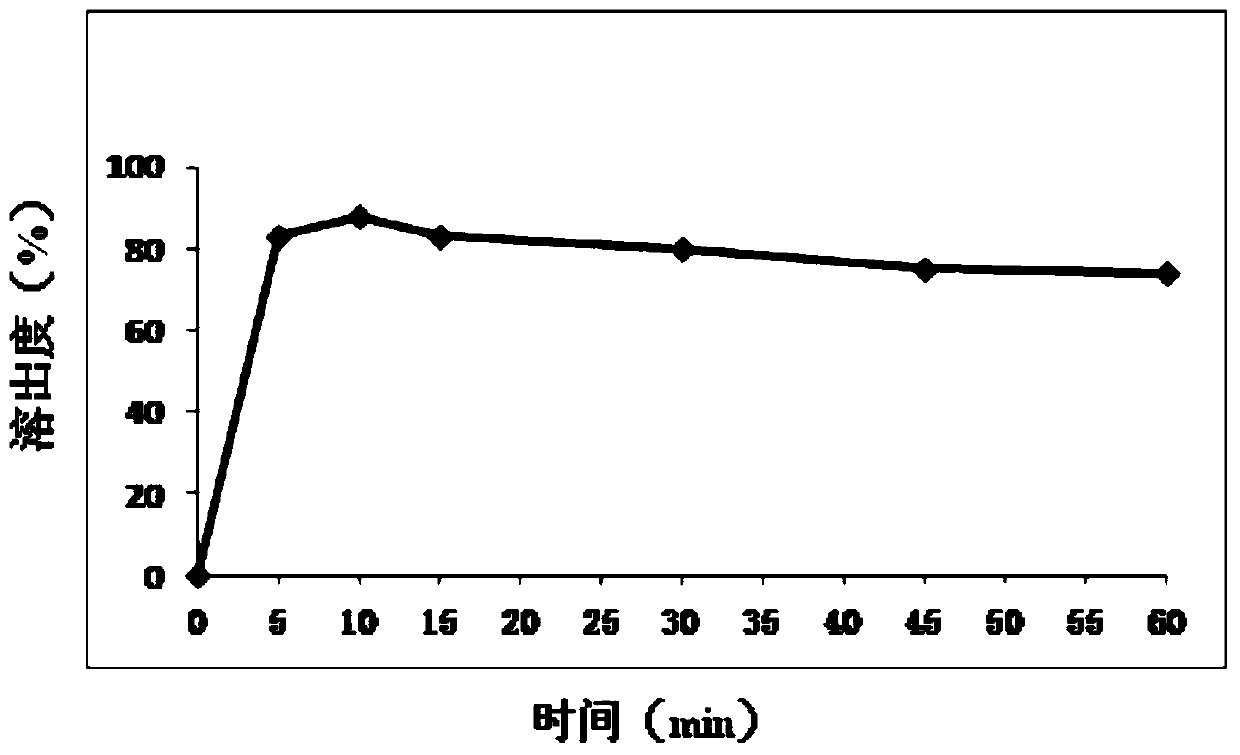
[0165] 1. Investigation on the particle size of Mesosulide API
[0166] Follow the steps below to investigate the impact of the particle size of the Mesosulide API on the Mesosulide Tablets, specifically as follows:
[0167] Based on the prescription a28 in Example 2, the preparation process of the present invention is further optimized in order to further improve the dissolution rate of the mesosulide film-coated tablet core of the present invention.
[0168] Specifically, according to prescription a28, two groups (group A and group B) of mesosulide film-coated tablet cores were prepared respectively. The method for preparing group A mesosulide film-coated tablet cores is as follows. The difference between the preparation method of Sully tablet and the preparation method of group A mesosulide film-coated tablet core is that the mixture of main drug mesosulide and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com