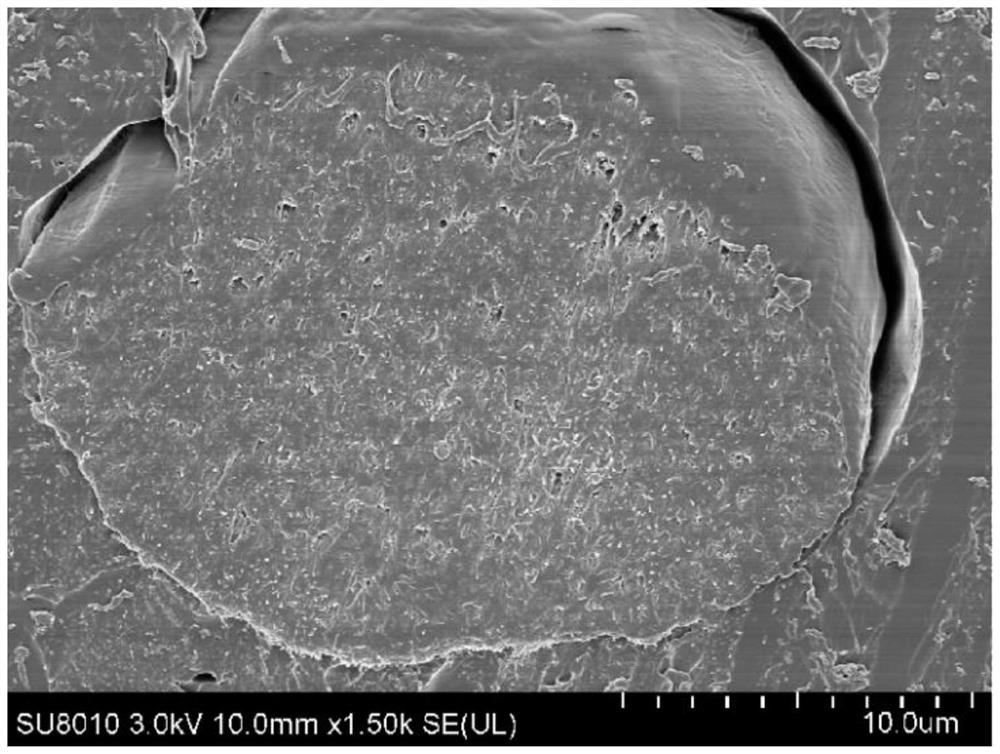
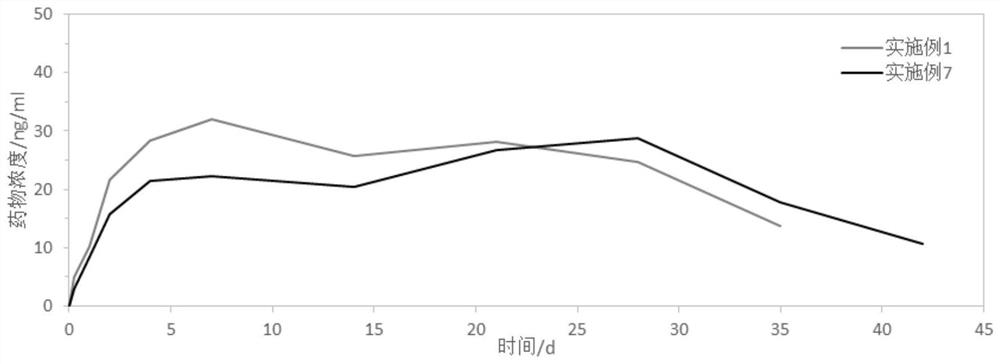
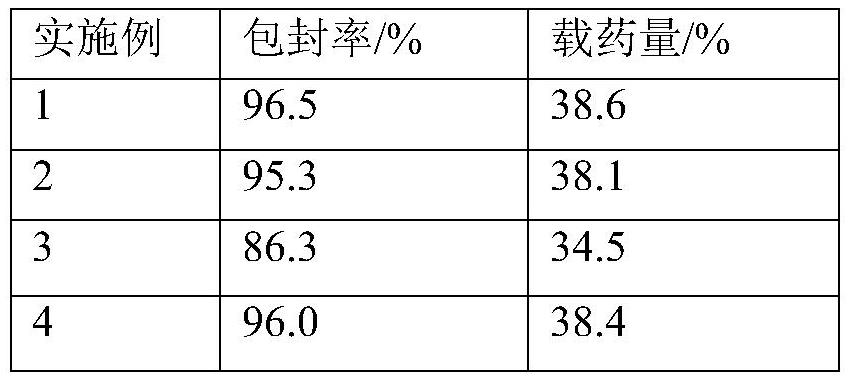
Aripiprazole sustained-release microsphere composition
A technology of aripiprazole and sustained-release microspheres, which is applied in drug combinations, nervous system diseases, organic active ingredients, etc., can solve the problems of poor storage stability, unsuitable for industrialization, and low proportion, and achieve stable release behavior , reduce pain, improve success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0050] An aripiprazole sustained-release microsphere composition, the starting materials for the preparation of the aripiprazole sustained-release microsphere composition include aripiprazole, acetic acid and a polymer, and the aripiprazole sustained-release microsphere composition is prepared. The final components of the ball composition are aripiprazole and polymer; wherein the molar ratio of acetic acid to aripiprazole is 2-5:1. The acetic acid is glacial acetic acid.
[0051] In the aripiprazole sustained-release microsphere composition, the weight content of aripiprazole is 30-45%, and the weight content of polymer is 55-70%. The polymer is selected from one or more of glycolide-lactide copolymer, polylactic-glycolic acid copolymer, polylactic acid, and polylactide.
[0052] The polymer is preferably a glycolide-lactide copolymer, and the molecular weight distribution of the glycolide-lactide copolymer is: 5-8% with a molecular weight ≥ 40000 Da, 15-17% with a molecular ...
Embodiment 1
[0058] 20.0g aripiprazole, 30.0g glycolide-lactide copolymer 7525 (Mw18000; molecular weight above 40000Da accounted for 6.73%, 25000 ~ 40000Da (excluding 40000, the same below) accounted for 15.77%, 17000 ~ 25000Da (no Including 25000, the same below) accounted for 39.82%, 10000 ~ 17000Da (excluding 17000, the same below) accounted for 22.01%, 5000 ~ 10000Da (excluding two endpoints, the same below) accounted for 10.11%, 5000Da or less accounted for 1.42 %) into 200.0 g of dichloromethane, stirred and dissolved at 32 °C for 1.5 h; then added 5.36 g of glacial acetic acid (2 times the molar ratio of aripiprazole), continued to stir and dissolve for 20 min to obtain an organic phase, and cooled to 25 °C for later use;
[0059] Add polyvinyl alcohol to 15L of water to a final concentration of 5g / L, stir and dissolve at 32°C to obtain an outer water phase, cool down to 25°C for later use;
[0060] The above-mentioned organic phase and external water phase were emulsified by an on...
Embodiment 2
[0063] 20.0g aripiprazole, 30.0g glycolide-lactide copolymer 7525 (Mw18000; molecular weight above 40000Da accounted for 5.42%, 25000~40000Da accounted for 15.01%, 17000~25000Da accounted for 38.54%, 10000~17000Da accounted for 22.92%, 5000-10000Da accounted for 11.301%, and below 5000Da accounted for 1.75%) into 200.0g of dichloromethane, stirred and dissolved at 32°C for 1.5h; then added 10.71g of glacial acetic acid (4 times the molar ratio of aripiprazole), Continue stirring and dissolving for 20min to obtain an organic phase, which is cooled to 25°C for later use;
[0064] Add polyvinyl alcohol to 15L of water to a final concentration of 5g / L, stir and dissolve at 32°C to obtain an outer water phase, cool down to 25°C for later use;
[0065] The above-mentioned organic phase and external water phase were emulsified by an online shearing machine at a ratio of 1:100 (v / v), and after emulsification, 25° C., 150 rpm was dried in liquid for 3 hours, and the solidified microsph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com