Subcutaneous implant line for long effect pressure lowering
An implanted thread, long-acting technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, cardiovascular system diseases, etc., can solve the side effects of inability to achieve long-term stable blood pressure, slow, and after a week In order to reduce blood pressure, increase patients' surgical pain and other problems, achieve the effect of reducing surgical pain, slow degradation, and no side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
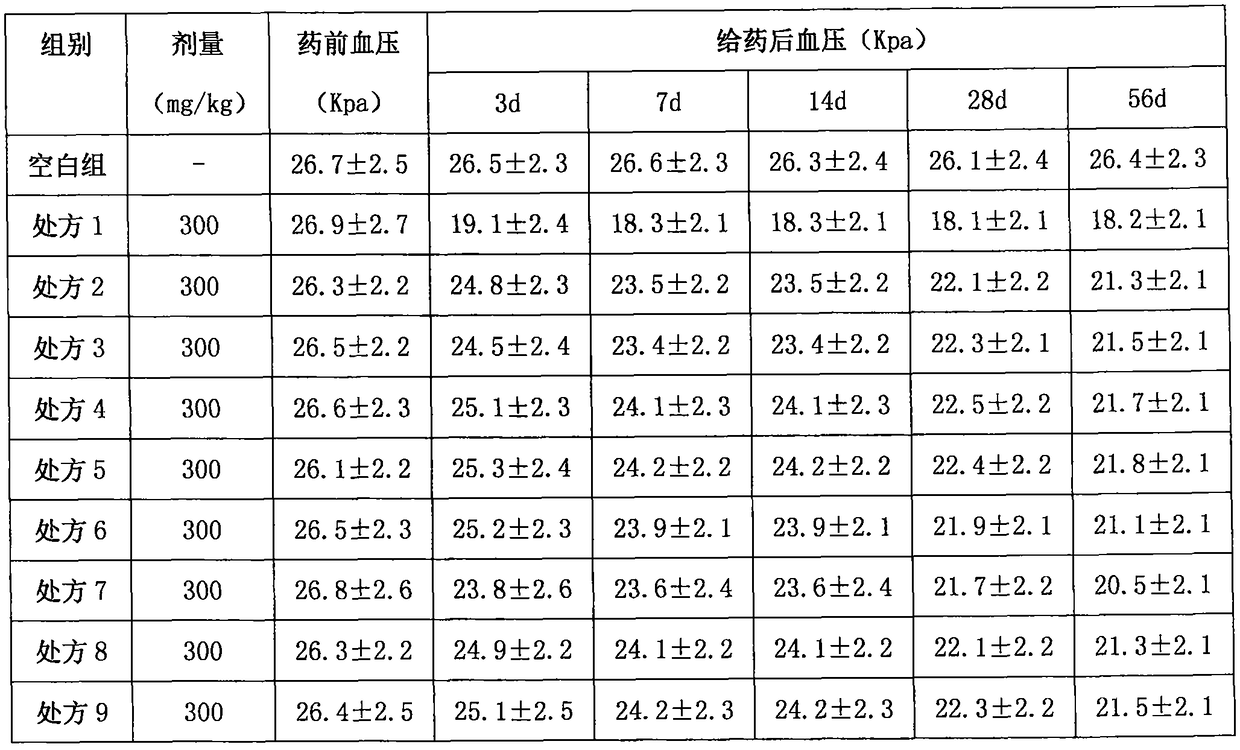
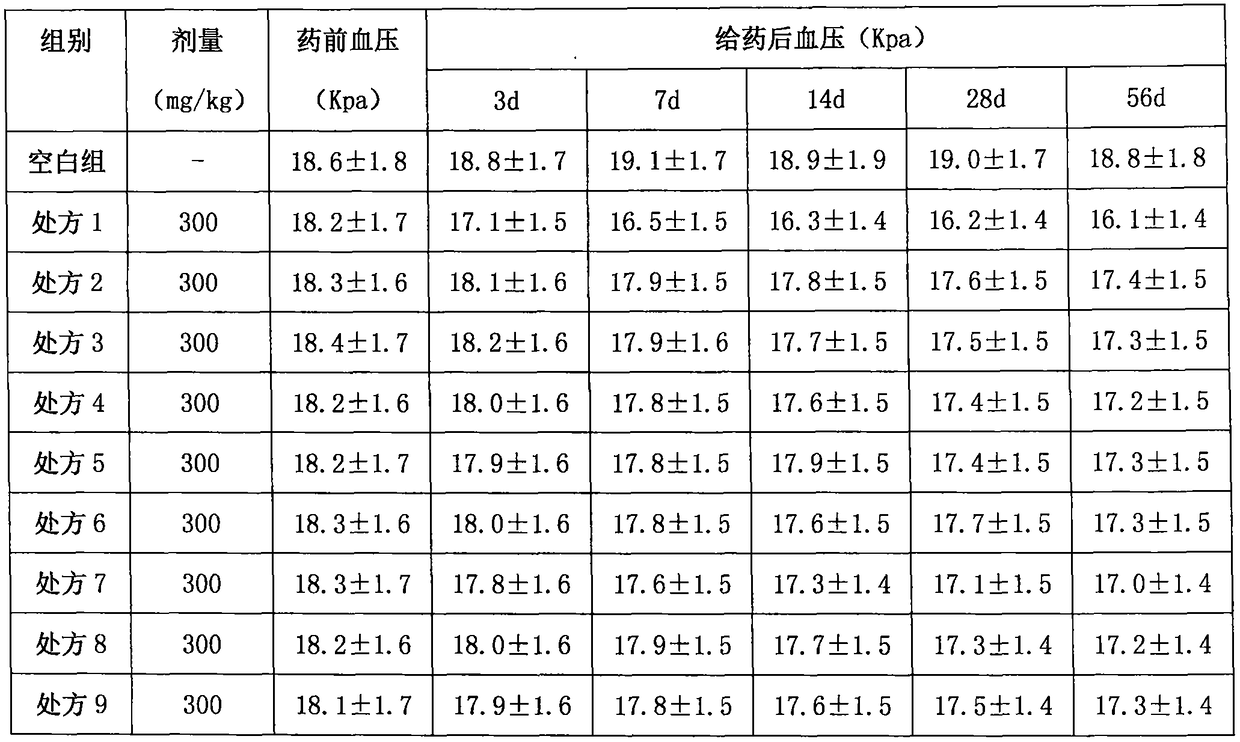
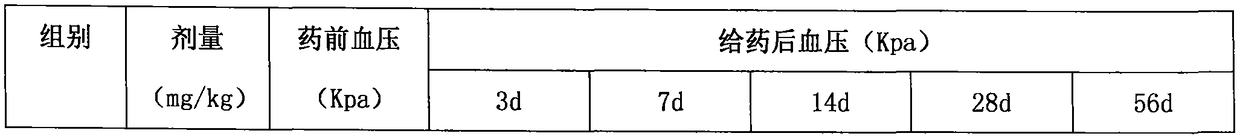
Image
Examples
Embodiment 1
[0167] API:
[0168] Lacidipine 6kg
[0169] Spironolactone 12kg
[0170] Indapamide 0.6kg;
[0171] Accessories:
[0172] Crospovidone 12kg
[0173] Polyethylene glycol 4kg
[0174] Carboxymethyl starch sodium 8kg.
[0175] Superfinely pulverize lacidipine, spironolactone and indapamide respectively to obtain fine powders for later use; put crospovidone and polyethylene glycol into the reaction kettle, heat at 130°C, and stir for 4 hours while heating , when the auxiliary materials in the reaction kettle are in a viscous state, add carboxymethyl starch sodium and continue to stir for 1 hour, and finally add lacidipine, indapamide and spironolactone superfine powder, and stir to obtain a mixture, mix The material is cooled and air-dried into a solid, pour the solid mixture into the extruder, extrude a cylindrical strip with a diameter of 2mm, and place the extruded cylindrical strip in a blast drying oven at 40°C for 7 hours, until the water evaporates completely Finall...
Embodiment 2
[0177] API:
[0178] Lacidipine 4kg
[0179] Spironolactone 15kg
[0180] Indapamide 0.4kg;
[0181] Accessories:
[0182] Crospovidone 16kg
[0183] Polyethylene glycol 3kg
[0184] Carboxymethyl starch sodium 11kg.
[0185] Superfinely pulverize lacidipine, spironolactone and indapamide respectively to obtain fine powders for later use; put crospovidone and polyethylene glycol into the reaction kettle, heat at 130°C, and stir for 4 hours while heating , when the auxiliary materials in the reaction kettle are in a viscous state, add carboxymethyl starch sodium and continue to stir for 1 hour, and finally add lacidipine, indapamide and spironolactone superfine powder, and stir to obtain a mixture, mix The material is cooled and air-dried into a solid, pour the solid mixture into the extruder, extrude a cylindrical strip with a diameter of 2mm, and place the extruded cylindrical strip in a blast drying oven at 40°C for 7 hours, until the water evaporates completely Final...
Embodiment 3
[0187] API:
[0188] Lacidipine 8kg
[0189] Spironolactone 9kg
[0190] Indapamide 0.8kg;
[0191] Accessories:
[0192] Crospovidone 8kg
[0193] Polyethylene glycol 7kg
[0194] Carboxymethyl starch sodium 5kg.
[0195]Superfinely pulverize lacidipine, spironolactone and indapamide respectively to obtain fine powders for later use; put crospovidone and polyethylene glycol into the reaction kettle, heat at 130°C, and stir for 4 hours while heating , when the auxiliary materials in the reaction kettle are in a viscous state, add carboxymethyl starch sodium and continue to stir for 1 hour, and finally add lacidipine, indapamide and spironolactone superfine powder, and stir to obtain a mixture, mix The material is cooled and air-dried into a solid, pour the solid mixture into the extruder, extrude a cylindrical strip with a diameter of 2mm, and place the extruded cylindrical strip in a blast drying oven at 40°C for 7 hours, until the water evaporates completely Finally, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com