Preparation method of lacidipine composition
A technology of lacidipine and composition, which is applied in the direction of drug combination, non-active ingredient medical preparations, medical preparations containing active ingredients, etc. It can solve the problems of low fat solubility, slow release and absorption, and unsatisfactory drug dissolution rate, etc. problem, to achieve the effect of increasing dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] The formula of lacidipine tablets is as follows, according to the dosage per 1000 tablets:
[0013] Lacidipine 4g;
[0014] Poloxamer 188 40g;
[0015] Polyvinylpyrrolidone (PVP) K30, 3g;
[0016] Crospovidone 2g;
[0017] Lactose 70g;
[0019] The preparation process is as follows: take 40g of poloxamer 188, heat it in a water bath at 65°C until it melts, take 4g of lacidipine raw material drug and disperse it in the melted poloxamer 188, stir until the lacidipine is completely dissolved, and then cool to 0°C Finally, crush (control the temperature not to exceed 35°C) and pass through a 120-mesh sieve, add polyvinylpyrrolidone (PVP) K30, crospovidone, and lactose, mix evenly, granulate with a 24-mesh sieve dry granulator, and granulate with a 24-mesh sieve Add the magnesium stearate of formula quantity, under 150MPa pressure, direct compression obtains the lacidipine sheet of embodiment 1.
Embodiment 2
[0021] The formula of lacidipine tablets is as follows, according to the dosage per 1000 tablets:
[0022] Lacidipine 4g;
[0023] Poloxamer 188 60g;
[0024] Polyvinylpyrrolidone (PVP) K30, 3g;
[0025] Crospovidone 2g;
[0026] Lactose 50g;
[0027] Magnesium stearate 1g.
[0028] The preparation process is as follows: take 60g of poloxamer 188, heat it in a water bath at 65°C until it melts, take 4g of lacidipine crude drug and disperse it in the melted poloxamer 188, stir until the lacidipine is completely dissolved, and then cool to 0°C Finally, crush (control the temperature not to exceed 35°C) and pass through a 120-mesh sieve, add polyvinylpyrrolidone (PVP) K30, crospovidone, and lactose, mix evenly, granulate with a 24-mesh sieve dry granulator, and granulate with a 24-mesh sieve Add the magnesium stearate of formula quantity, under 150MPa pressure, direct compression obtains the lacidipine tablet of embodiment 2.
Embodiment 3
[0030] The formula of lacidipine tablets is as follows, according to the dosage per 1000 tablets:
[0031] Lacidipine 6g;
[0032] Poloxamer 188 60g;
[0033] Polyvinylpyrrolidone (PVP) K30, 3g;
[0034] Crospovidone 2g;
[0035] Lactose 70g;
[0036] Magnesium stearate 1g.
[0037] The preparation process is as follows: take 40g of poloxamer 188, heat it in a water bath at 65°C until it melts, take 6g of lacidipine raw material drug and disperse it in the melted poloxamer 188, stir until the lacidipine is completely dissolved, and then cool to 0°C Finally, crush (control the temperature not to exceed 35°C) and pass through a 120-mesh sieve, add polyvinylpyrrolidone (PVP) K30, crospovidone, and lactose, mix evenly, granulate with a 24-mesh sieve dry granulator, and granulate with a 24-mesh sieve Add the magnesium stearate of formula quantity, under 150MPa pressure, direct compression obtains the lacidipine sheet of embodiment 3.
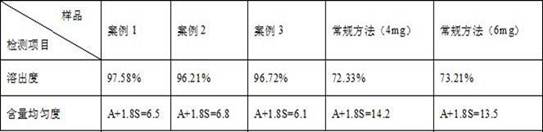
[0038] By comparing the lacidipine prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com