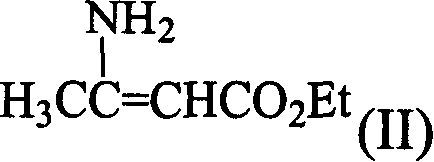Process for synthesizing lacidipine of antihypertensive drugs
A technology of lacidipine and its compounds, which is applied in the field of synthetic technology of antihypertensive drug lacidipine, can solve problems such as low yield, long route, and no practical production significance, and achieve high yield, low cost, and easy separation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
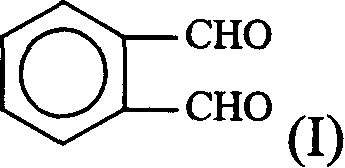
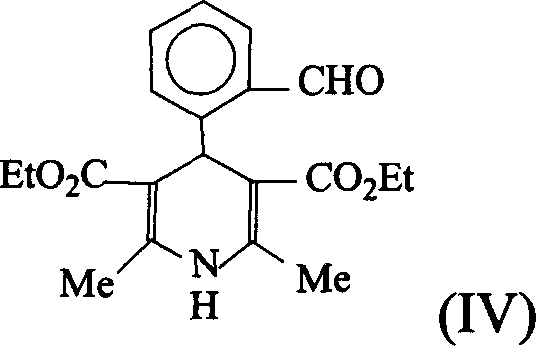
[0047] In a 500ml round bottom flask, add 13.4g (0.1mol) of o-phthalaldehyde, 100ml of ethyl acetate, 23.2g (0.2mol) of ethyl aminocrotonate, cool in an ice-water bath, and add 50ml of glacial acetic acid dropwise with stirring. After reacting at 0-5°C for 4 hours, add 250ml of ethyl acetate and 50ml of water, separate the water layer, wash the organic layer with 2×50ml of water, wash once with 50ml of saturated NaCl aqueous solution, and wash with anhydrous NaCl. 2 SO 4 Dry and concentrate to obtain a brown oil, which was placed in the refrigerator; the next day, crystals were precipitated, filtered, and washed with a small amount of cold ethyl acetate to obtain compound (IV), 17.85 g of yellow crystals, m.p.177-179°C, yield 50%. 1 H-NMR (CDCl 3 , 60MHz) ppm: 1.18 (6H, triplet); 2.3 (6H, singlet); 4.0 (4H, quartet); 5.8 (1H, singlet); 6.25 (1H, singlet); 7.17.9 ( 4H, multiplet).
Embodiment 2
[0049] In a 250ml round bottom flask, add 3.57g (0.01mol) of compound (IV), 5.24g (0.02mol) of phosphate (III) (R = ethyl), 150ml of absolute ethanol, and cool with ice water at 0-5°C , add dropwise 20ml ethanol solution containing 0.02mol sodium ethoxide under stirring, finish adding in 30 minutes, then react at room temperature for 5 hours, stop stirring, evaporate about 100ml ethanol under reduced pressure, add 100ml water, precipitate yellow solid, filter, use Ethyl acetate was recrystallized to obtain 3.64 g of the product lacidipine as yellow-white crystals, m.p 175-177°C, and the yield was 80%. 1 H NMR (CDCl 3 , 90M Hz) ppm: 1.1 (6H, triplet); 1.5 (9H, singlet); 2.3 (6H, singlet); 4.0 (4H, multiplet); 5.4 (1H, singlet); 6.3 (1H, J=13 Hz, doublet); 6.5 (1H, singlet); 7.1-7.6 (4H, multiplet); 8.5 (1H, J=13Hz, doublet).
Embodiment 3
[0051] In a 100ml round-bottomed flask, add 39g of tert-butyl bromoacetate, dropwise add 35g of triethyl phosphite at 110°C, finish adding in 1 hour, then react for 3 hours, stop; distill under reduced pressure to obtain compound (III) (R =ethyl) 45.0g, b.p 122~124°C / 3mm Hg, yield 83%. 1 HNMR (CDCl 3, 300 MHz) ppm: 1.3 (6H, triplet); 1.4 (9H, singlet); 2.85 (2H, doublet); 4.15 (4H, quartet).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com