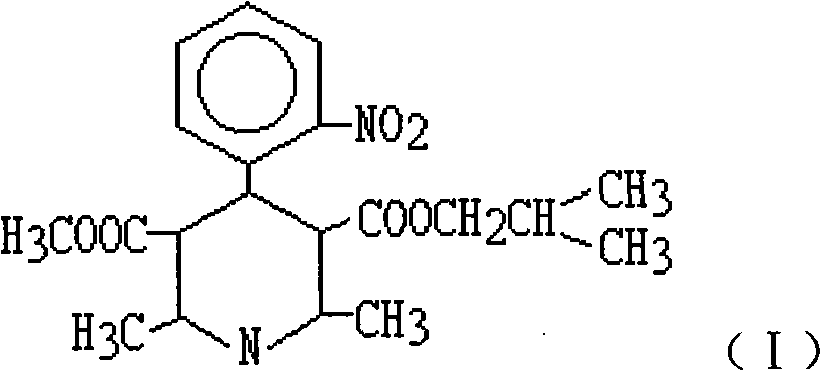Synthetic method of nisoldipine photolytic product reference substance
A synthetic method and technology of reference substances, applied in the direction of organic chemistry, etc., can solve the problems of long synthetic route, many reagents used, labor and environmental damage, etc., and achieve the effect of less environmental pollution, less toxic effect, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Nisoldipine 40g was made into a saturated solution of absolute ethanol. At room temperature, in an airtight container, the color changes from yellow to dark blue-green after three days of sunlight exposure. The solution was refluxed in a water bath for 30 minutes at a temperature of 70 degrees Celsius; the insoluble matter was filtered off while it was hot, and the filtrate was dried under reduced pressure at 50 degrees Celsius with a rotary evaporator. The resulting residue was recrystallized three times with absolute ethanol to obtain a refined product as a light blue-green powder.
example 2
[0026] Nisoldipine 80g was made into a saturated solution of absolute ethanol. At room temperature, in a closed container, after being irradiated by infrared light for 2 days, the color turns from yellow to dark blue-green. The solution was refluxed in a water bath for 20 minutes at a temperature of 75 degrees Celsius; while it was hot, the insoluble matter was filtered off with suction, and the filtrate was dried under reduced pressure at 60 degrees Celsius with a rotary evaporator. The resulting residue was recrystallized three times with absolute ethanol to obtain a refined product as a light blue-green powder.
example 3
[0028] Nisoldipine 70g was made into a saturated solution of absolute ethanol. At room temperature, in an airtight container, the color changes from yellow to dark blue-green after four days of exposure to sunlight. The solution was refluxed in a water bath for 50 minutes at a temperature of 80 degrees Celsius; the insoluble matter was filtered off while it was hot, and the filtrate was dried under reduced pressure at 70 degrees Celsius with a rotary evaporator. The resulting residue was recrystallized twice with ... to obtain the refined product as light blue-green powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com