Nisoldipine capsule and preparation method thereof
A technology of dipine capsules and nisoldipine, applied in the field of medicine, can solve the problems of complex preparation process, low dissolution rate, poor absorption, etc., and achieve the effects of stable drug release, small difference in tablet weight, and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0033] Nisoldipine, filler, sodium lauryl sulfate and magnesium stearate were pulverized through a 60-mesh sieve; 400 g of nisoldipine, 8800 g of filler and 120 g of sodium lauryl sulfate were weighed and mixed. Weigh 50 g of povidone and add absolute ethanol to prepare a 6% solution as a binder. Nisoldipine, mannitol and sodium lauryl sulfate are added to a wet granulator, and the povidone solution is used as a binder to make soft materials, and granulated with a 20-mesh screen. Dry using a fluidized granulation dryer. The granules were sized with a 20-mesh sieve in a swing granulator, and 9370 g of the granules and 64 g of magnesium stearate were added to the mixer and mixed for 10 minutes. Capsule filling with capsule filling machine.
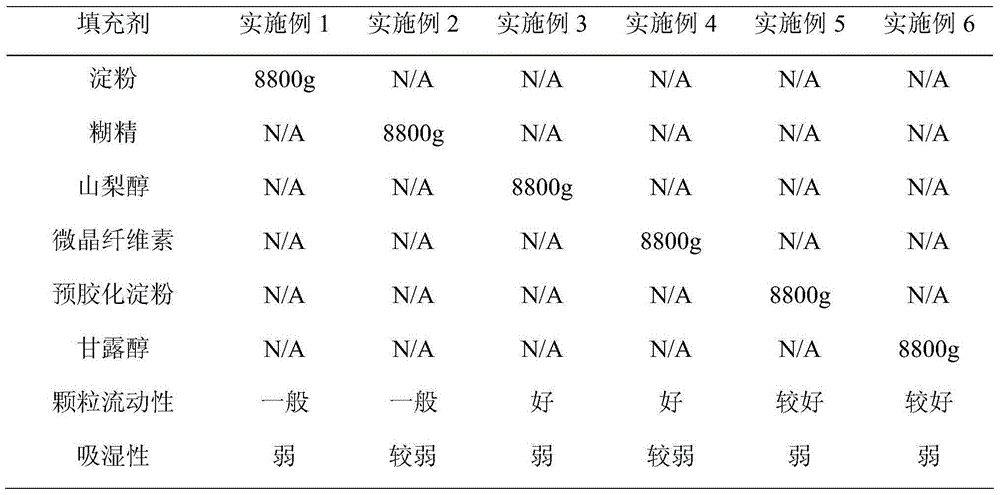
[0034] See Table 1 for the types and amounts of fillers used in Examples 1-6 and related performance indicators.
[0035] As can be seen from the data in Table 1, Example 3 has the best performance.
[0036] The kind and consumption of t...
Embodiment 7-9
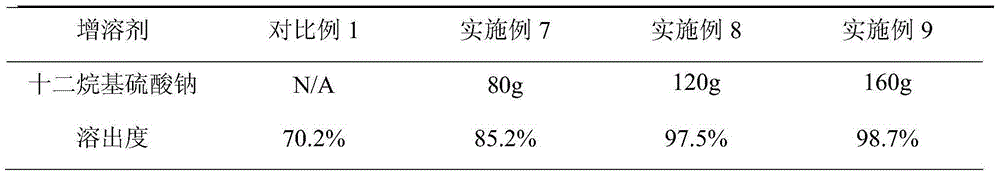
[0039] Nisoldipine, filler, sodium lauryl sulfate and magnesium stearate were pulverized through a 60-mesh sieve; 400 g of nisoldipine, 8800 g of sorbitol and a solubilizer were weighed, and mixed evenly. Weigh 50 g of povidone and add absolute ethanol to prepare a 6% solution as a binder. Nisoldipine, mannitol and solubilizer are added to the wet granulator, and the povidone solution is used as a binder to make soft materials, and granulated with a 20-mesh screen. Dry using a fluidized granulation dryer. The granules were sized with a 20-mesh sieve in a swing granulator, and 9370 g of the granules and 64 g of magnesium stearate were added to the mixer and mixed for 10 minutes. Capsule filling with capsule filling machine.
Embodiment 10
[0047] Nisoldipine, fillers, sodium lauryl sulfate and magnesium stearate were pulverized through a 60-mesh sieve; 400 g of nisoldipine, 8800 g of sorbitol and 120 g of sodium lauryl sulfate were weighed and mixed. Add nisoldipine, mannitol and solubilizer to a wet granulator, add a soft material made of a binder, and granulate with a 20-mesh sieve. Dry using a fluidized granulation dryer. The granules were sized with a 20-mesh sieve in a swing granulator, and 9370 g of the granules and 64 g of magnesium stearate were added to the mixer and mixed for 10 minutes. Capsule filling with capsule filling machine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com