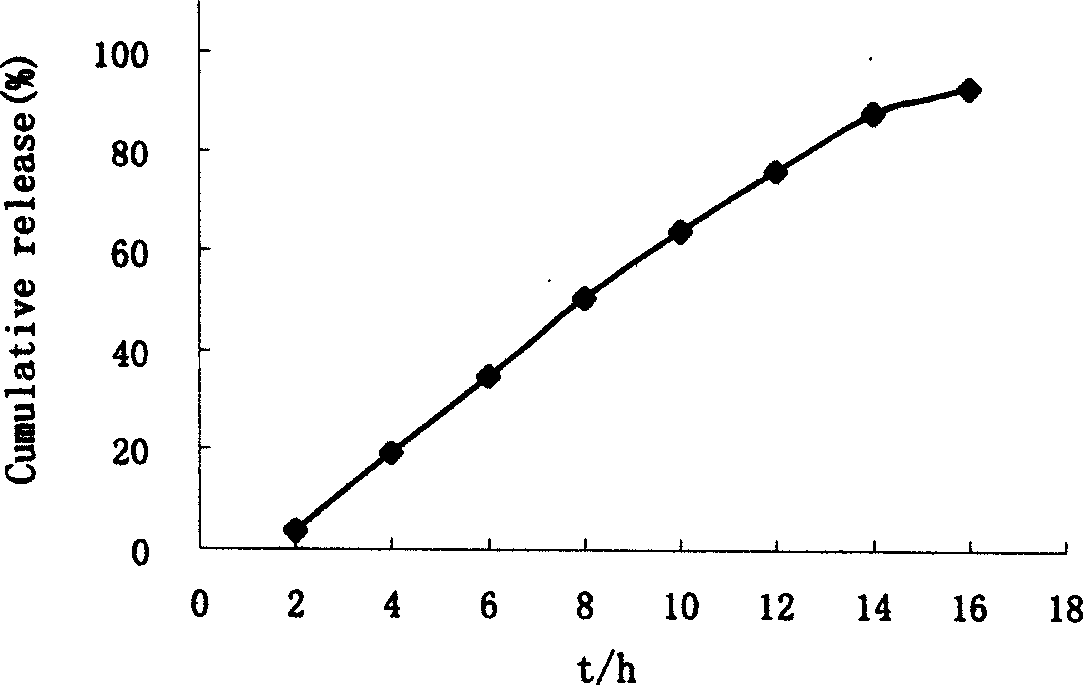
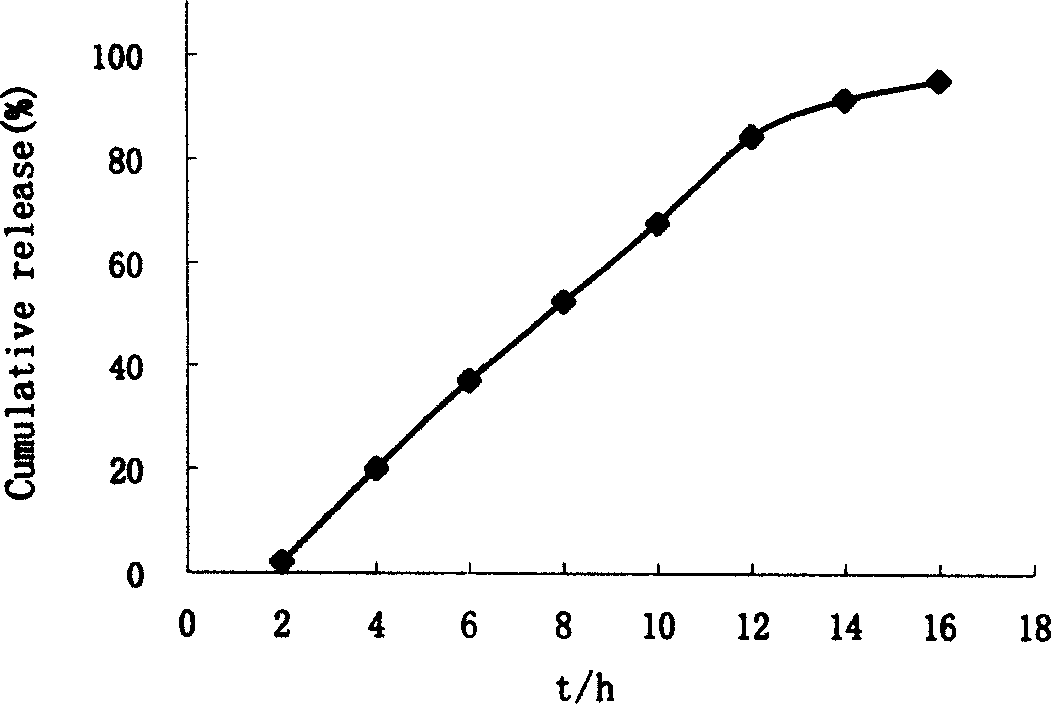
Nisoldipine double layer penetrated pump control releasing tablets
A technology of osmotic pump controlled release and nisoldipine, which is applied in the fields of cardiovascular system diseases, pharmaceutical formulations, drug combinations, etc., can solve the problems of frequent taking of ordinary tablets, long-lasting drug effects, large fluctuations in blood drug concentration, etc., to achieve The effect of long-lasting drug effect, small fluctuation of blood drug concentration, and less frequency of taking medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The present embodiment 1 tablet that adopts the known method of pharmaceutical industry to make contains following composition by weight percentage:
[0020] Drug-containing layer:
[0021] Nisoldipine 5.5%
[0022] Polyoxyethylene (molecular weight: 100,000) 50.0%
[0023] Magnesium Stearate 0.2%
[0024] 10% PVP ethanol solution appropriate amount
[0025] Boost layer:
[0026] Polyoxyethylene (molecular weight 7 million) 27.8%
[0027] Hypromellose 1.4%
[0029] Povidone (K30) 2.8%
[0030] Magnesium Stearate 0.2%
[0031] 10% PVP ethanol solution appropriate amount
[0032] Composition of semi-permeable membrane coating solution:
[0033] Cellulose acetate 96.5%
[0034] Macrogol 4000 3.5%
[0035] Acetone: water 3.0% (w / v)
[0036] Composition of moisture-proof coating solution:
[0037] Hypromellose 42.9%
[0038] 1,2-Propanediol 2.0% (v / v)
[0039] Talc 28.6%
[0040] Titanium dioxide 28.6%
[0041] Ethanol: water ...
Embodiment 2
[0044] The present embodiment 1 tablet that adopts the known method of pharmaceutical industry to make contains following composition by weight percentage:
[0045] Drug-containing layer:
[0046] Nisoldipine 5.3%
[0047] Polyoxyethylene (molecular weight: 200,000) 42.1%
[0049] Magnesium Stearate 0.2%
[0050] 10% PVP ethanol solution appropriate amount
[0051] Boost layer:
[0052] Polyoxyethylene (molecular weight: 5 million) 26.1%
[0054] Povidone (K30) 2.8%
[0055] Magnesium Stearate 0.2%
[0056] 10% PVP ethanol solution appropriate amount
[0057] Composition of semi-permeable membrane coating solution:
[0058] Cellulose acetate 91.3%
[0059] Triethyl Citrate 6.3%
[0060] Macrogol 400 2.4%
[0061] Acetone: water 3.6% (w / v)
[0062] Nisoldipine is made into a double-layer osmotic pump controlled-release tablet by film coating technology, and the drug-containing layer is drilled by laser o...
Embodiment 3
[0064] The present embodiment 1 tablet that adopts the known method of pharmaceutical industry to make contains following composition by weight percentage:
[0065] Drug-containing layer:
[0066] Nisoldipine 5.5%
[0067] Carbopol 40.1%
[0068] Lactose 12.6%
[0069] Talc 0.2%
[0070] Boost layer:
[0071] Polyoxyethylene (molecular weight 6 million) 29.0%
[0072] Lactose 9.5%
[0073] Talc 0.2%
[0074] 10% PVP ethanol solution appropriate amount
[0075] Composition of semi-permeable membrane coating solution:
[0076] Cellulose acetate 80.7%
[0077] Ethylcellulose 9.2%
[0078] Methyl phthalate 10.1%
[0079] Acetone: water 2.1% (w / v)
[0080] Nisoldipine is made into a double-layer osmotic pump controlled-release tablet by film coating technology, and the drug-containing layer is drilled by laser or mechanically to achieve the purpose of controlled release.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com