Eye drops containing tafluprost and preparation method thereof
A technology of tafluprost and eye drops, applied to medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., which can solve the problem of unstable intraocular pressure effects, inaccurate doses, and increased risks of inducing toxic and side effects And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
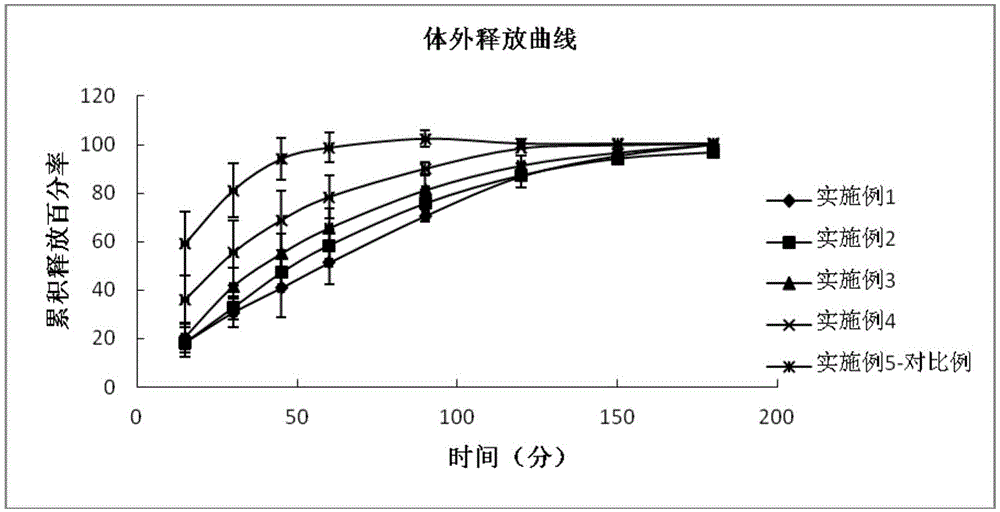
[0011] The inventors have found in their research that using the hydrophilic polymer polycarbophil AA-1 or carbomer as an eye drop sustained-release material, which is in the form of an aqueous solution in an in vitro environment, when dripped into the conjunctival sac, Affected by the temperature, pH or ionic strength of the eye, it quickly forms a gel and attaches to the surface of the eyeball, forming a very thin layer of drug storage, which can not only increase the retention time of the drug in the eye, avoid the rapid loss of the drug, and The formed drug depot has the effect of slowly releasing the drug, avoiding the rapid elimination of the drug, and improving the bioavailability.
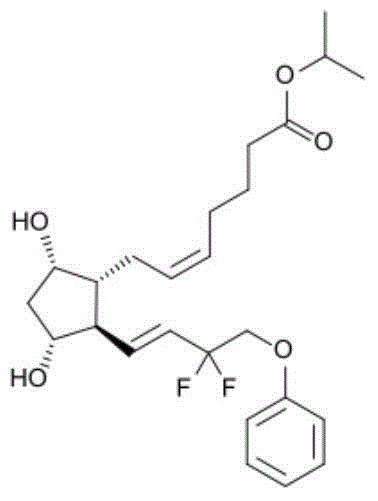
[0012] The invention provides an eye drop containing tafluprost, which comprises the active ingredient tafluprost and a hydrophilic polymer, wherein the hydrophilic polymer is selected from polycarbophil or carbomer.
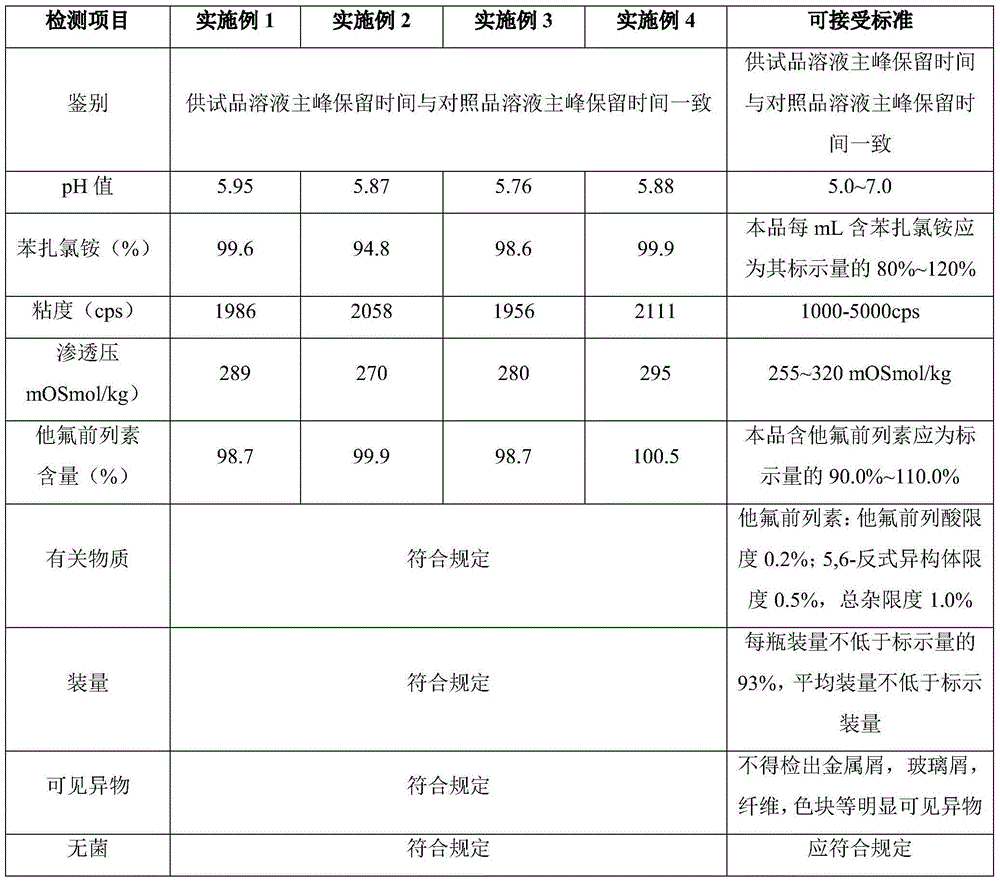
[0013] In some embodiments, according to the total weight percentage, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com