A kind of preparation method of nisoldipine impurity
A nisoldipine and impurity technology, which is applied in the field of preparation of nisoldipine impurities, can solve the problems of no drug efficacy and toxicity of the product, poor stability of nisoldipine, etc., and achieve the effects of high yield, low production cost, and simple processing method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Take 1g of nisoldipine, add 10mL of ethanol to dissolve;
[0032] (2) Add 15 mg of free radical initiator 2,2-2,2-azobisisobutyronitrile (AIBN) to the solution to obtain a reaction solution;
[0033] (3) Place the reaction solution under a fluorescent lamp with a power of 30w, irradiate and stir for 35h;
[0034] (4) Add 100 mL of water to the solution obtained in step (3), filter out the solid, and separate the product from the filtrate by column chromatography.
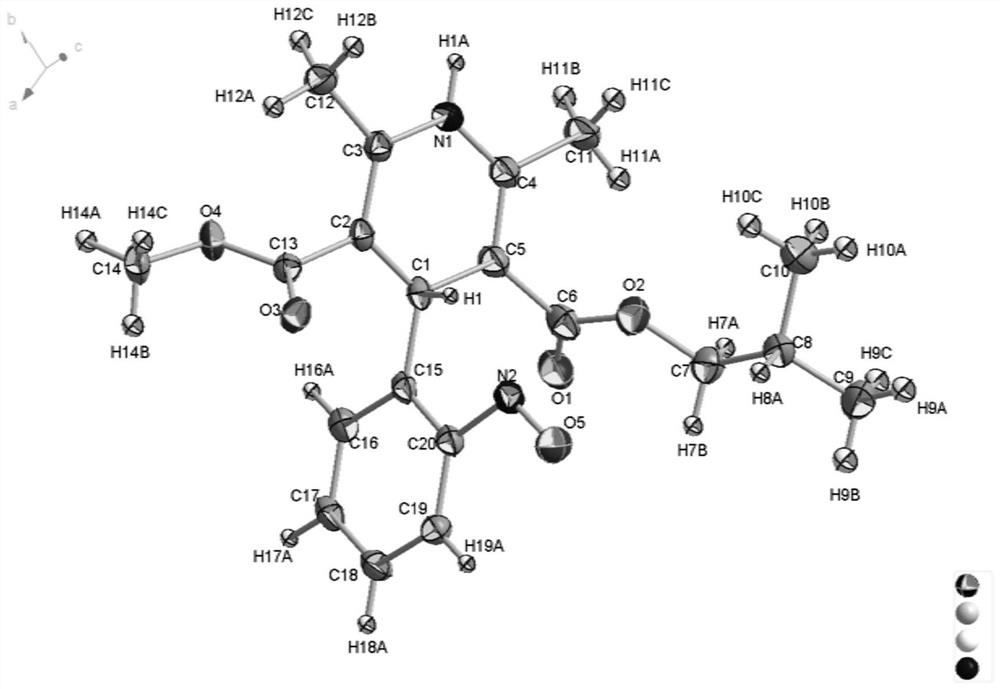
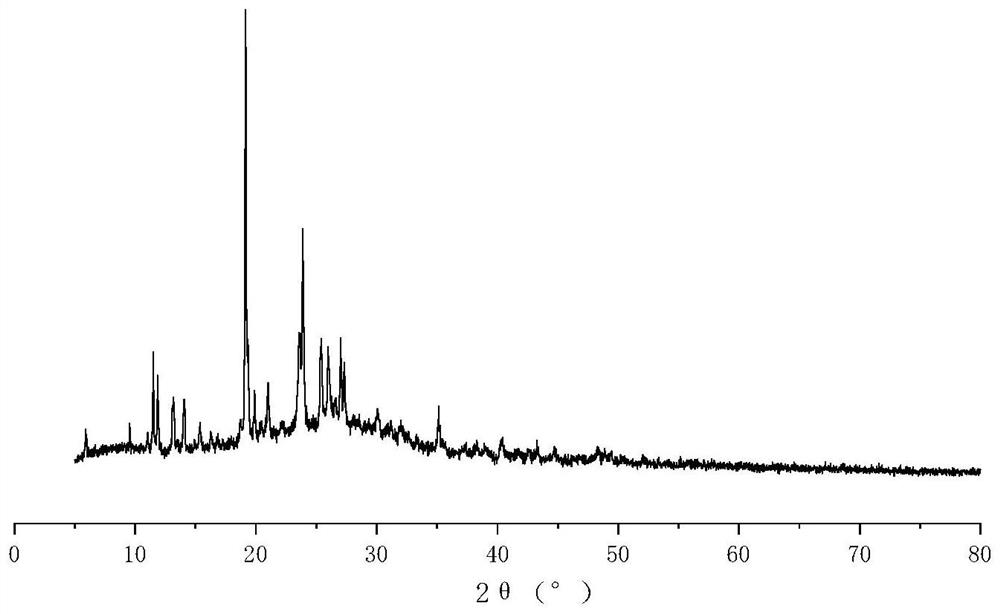
[0035] The solid sample of the product obtained in Example 1 above was dissolved in ethanol and left to stand for 7 days, and a blue-green prismatic single crystal was precipitated. The single crystal is subjected to X-ray single crystal diffraction, and the obtained crystal structure is as follows figure 1 As shown, the crystal structure belongs to the triclinic crystal system, the space group is P-1, (4), α / °=90.963 (2) β / °=98.659 (2) γ / °=108.680 (2) V=941.26 (4) Z=2.
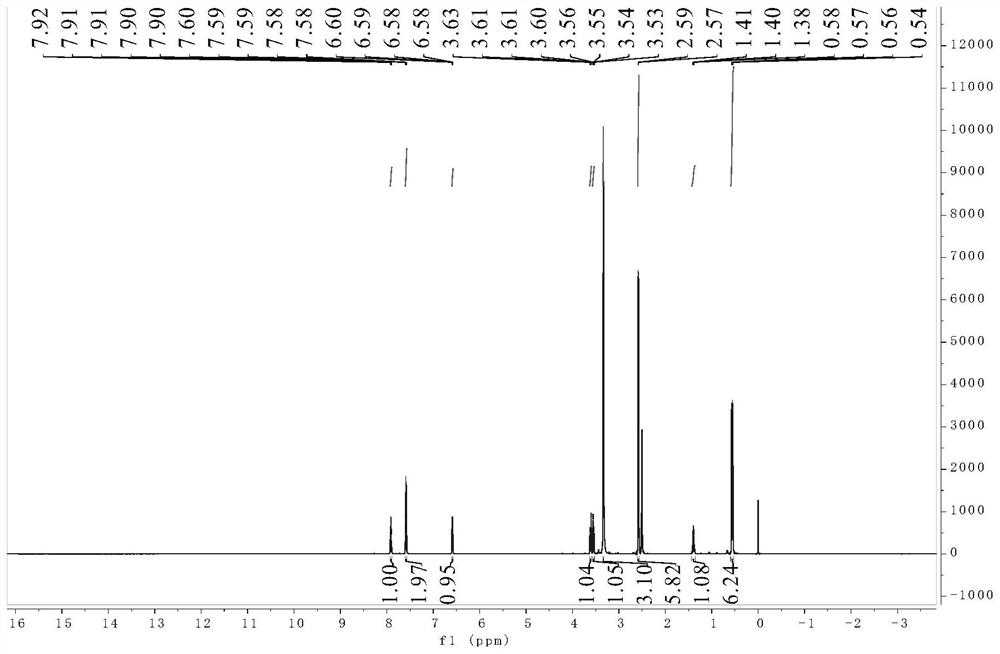
[0036] The product obtained ...
Embodiment 2
[0041] (1) Take 1g nisoldipine, add 20mL acetone to dissolve;
[0042] (2) 15 mg of free radical initiator 1,2,4,5-tetracyanobenzene (TCNB) was added to the solution to obtain a reaction solution;
[0043] (3) Place the reaction solution under a fluorescent lamp with a power of 30w, irradiate and stir for 15h;
[0044] (4) Add 100 mL of water to the solution obtained in step (3), filter out the solid, and separate the product from the filtrate by column chromatography.
Embodiment 3
[0046] (1) Take 1g of nisoldipine, add 5mL of ethanol and 10mL of acetone to dissolve;
[0047] (2) Add 10 mg of free radical initiator 2,2-2,2-azobisisobutyronitrile (AIBN) to the solution to obtain a reaction solution;
[0048] (3) Place the reaction solution under a fluorescent lamp with a power of 30w, irradiate and stir for 5h;
[0049] (4) Add 100 mL of water to the solution obtained in step (3), filter out the solid, and separate the product from the filtrate by column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com