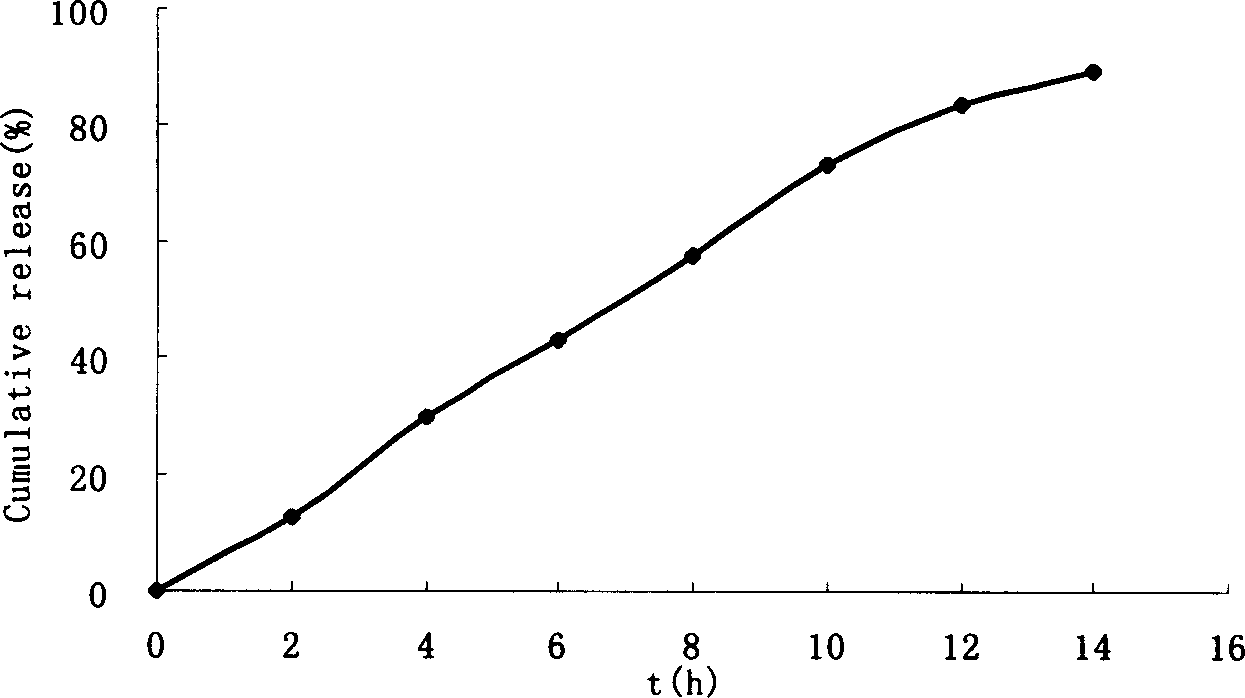
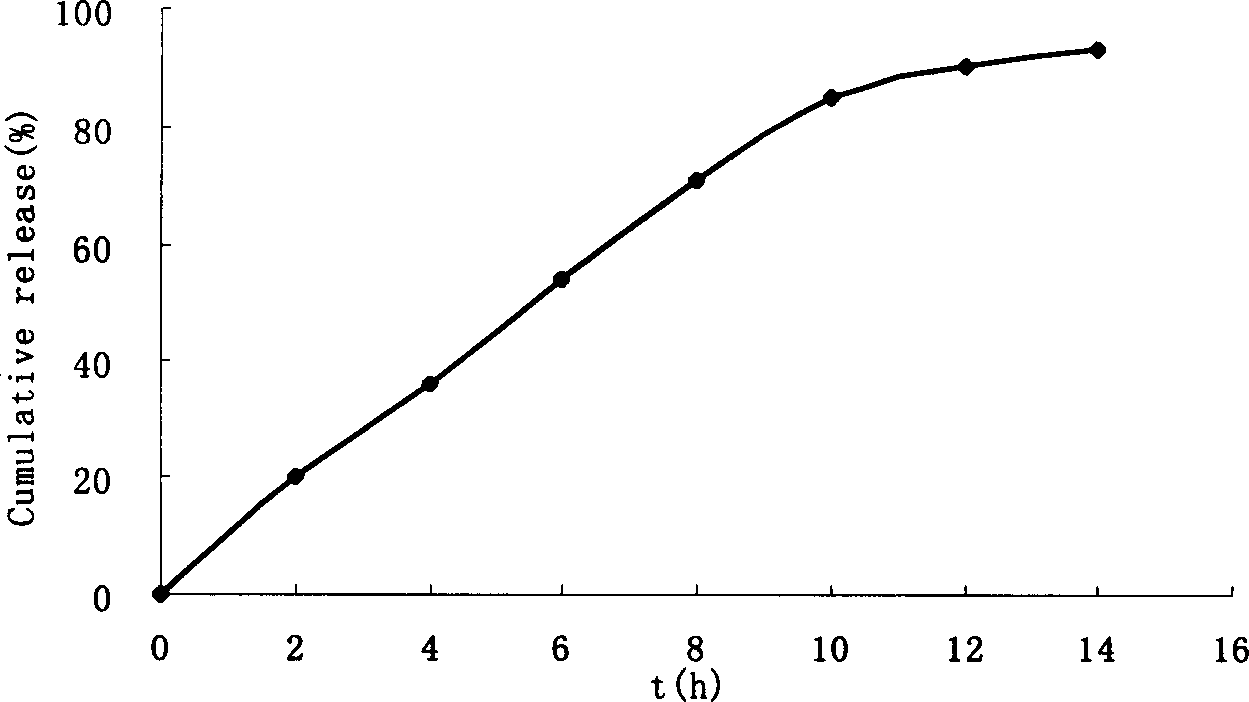
Monolayer osmotic pump controlled releasing tablets of nimodipine
An osmotic pump controlled-release, dipine monolayer technology, applied in the field of medicine, can solve the problems of many times of taking medicines, inconvenient taking, large fluctuations in blood drug concentration, etc., and achieve the effects of stable curative effect, long-lasting effect, and small toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] This example was prepared using methods known in the pharmaceutical industry. The tablet contains the following ingredients by weight percentage:
[0018] Tablet composition:
[0019] Nisoldipine 2.9%
[0020] Polyoxyethylene (molecular weight: 300,000) 48.3%
[0022] Magnesium Stearate 0.5%
[0023] 10% povidone K30 ethanol solution appropriate amount
[0024] Composition of semi-permeable membrane coating solution:
[0025] Cellulose acetate 90.0%
[0026] Macrogol 400 10.0%
[0027] Acetone: Water 3.0% (w / v)
[0028] Composition of moisture-proof coating solution:
[0029] Hypromellose 42.9%
[0030] 1,2-Propanediol 2.0% (v / v)
[0031] Talc 28.6%
[0032] Titanium dioxide 28.6%
[0033] Ethanol: Water 7.0% (w / v)
[0034] Using film coating technology, nisoldipine is made into single-layer osmotic pump controlled-release tablets, and one or both sides are laser or mechanically perforated to achieve the purpose of controlled...
Embodiment 2
[0036] This example was prepared using methods known in the pharmaceutical industry. The tablet contains the following ingredients by weight percentage:
[0037] Tablet composition:
[0038] Nisoldipine 2.9%
[0039] Polyoxyethylene (molecular weight: 200,000) 43.2%
[0040] Sodium chloride 43.4%
[0041] Starch 10.1%
[0042] Magnesium Stearate 0.4%
[0043] 10% povidone K30 ethanol solution appropriate amount
[0044] Composition of semi-permeable membrane coating solution:
[0045] Cellulose acetate 85.1%
[0046] Polyethylene glycol 200 14.9%
[0047] Acetone: Water 3.6% (w / v)
[0048] Moisture-proof coating liquid composition: with example 1
[0049] Using film coating technology, nisoldipine is made into single-layer osmotic pump controlled-release tablets, and one or both sides are laser or mechanically perforated to achieve the purpose of controlled release.
Embodiment 3
[0051] This example was prepared using methods known in the pharmaceutical industry. The tablet contains the following ingredients by weight percentage:
[0052] Tablet composition:
[0053] Nisoldipine 3.0%
[0054] Gum Arabic 34.1%
[0055] Sodium chloride 50.3%
[0056] Starch 11.1%
[0057] Magnesium Stearate 1.5%
[0058] 10% povidone K30 ethanol solution appropriate amount
[0059] Composition of semi-permeable membrane coating solution:
[0060] Cellulose acetate 85.0%
[0061] Dibutyl phthalate 15.0%
[0062] Acetone: Water 3.6% (w / v)
[0063] Moisture-proof coating liquid composition: with example 1
[0064] Using film coating technology, nisoldipine is made into single-layer osmotic pump controlled-release tablets, and one or both sides are laser or mechanically perforated to achieve the purpose of controlled release.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com