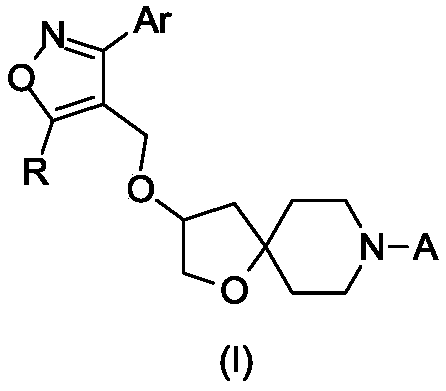
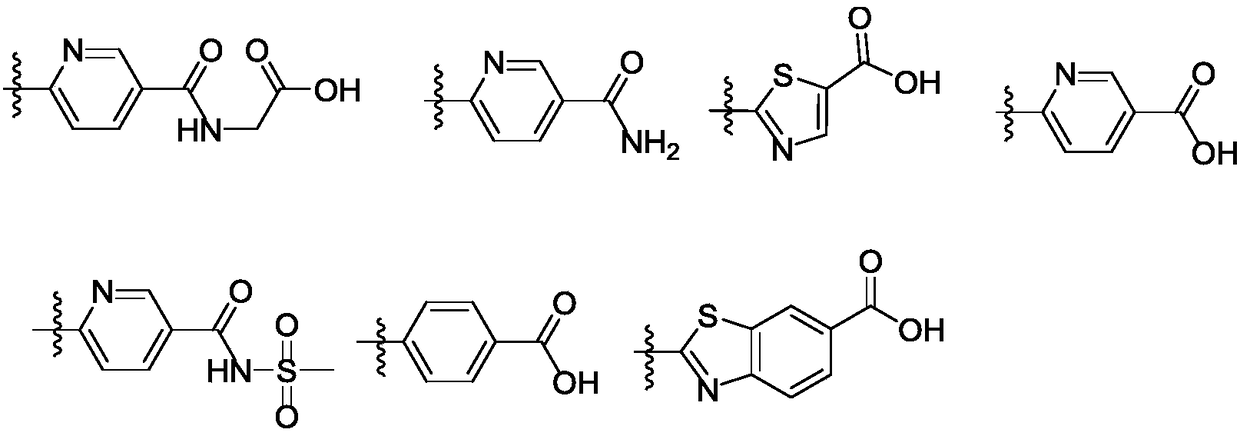
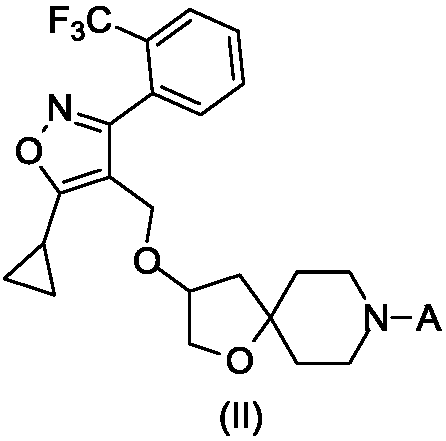
FXR regulator with spirane structure
A technology of stereoisomers and compounds, applied in the field of medicinal chemistry, can solve problems such as differential nuclear receptor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: 4-(3-((5-cyclopropyl-3-(2-(trifluoromethyl)phenyl)isoxazol-4-yl)methoxy)-1-oxa-8 -Azaspiro[4.5]decane-8-yl)benzoic acid
[0061] The synthesis steps are as follows:
[0062]
[0063] Step 1: Preparation of (E)-2-(trifluoromethyl)benzaldehyde oxime:
[0064]
[0065] NH at -5℃ 2 A solution of OH·HCl (8.0 g, 115 mmol) in water (15 mL) was slowly dropped into a solution of NaOH (4.8 g, 120 mmol) in water (15 mL). After stirring for 15 min, a solution of 2-(trifluoromethyl)benzaldehyde (17.4 g, 100 mmol) in ethanol (15 mL) was slowly added dropwise and the system was kept at -5°C. The reaction system became turbid, and the stirring was continued until the system became clear, and the system was slowly warmed to room temperature. After reacting at room temperature for 1 hour, TLC monitored the consumption of raw materials. The reaction system was diluted with water, extracted with EA three times, the organic layers were combined, washed with saturated brine, and anhy...
Embodiment 2
[0092] Example 2: 2-(3-((5-cyclopropyl-3-(2-(trifluoromethyl)phenyl)isoxazol-4-yl)methoxy)-1-oxa-8 -Azaspiro[4.5]decane-8-yl)benzo[d]thiazole-6-carboxylic acid
[0093] The synthesis steps are as follows:
[0094]
[0095] Step 1: Preparation of 2-aminobenzo[d]thiazole-6-carboxylic acid methyl ester
[0096]
[0097] Bromine (3.10g, 19.85mmol) was dissolved in acetic acid (18mL) and slowly added dropwise to methyl 4-aminobenzoate (3.00g, 19.85mmol), KSCN (6.89g, 70.86mmol) in acetic acid (67mL) In the solution, react overnight at room temperature. Quench with water, use saturated Na 2 CO 3 Adjust the pH of the solution to 9. Extract three times with EA, wash with saturated brine, anhydrous Na 2 SO 4 After drying and concentration, the crude product was passed through column chromatography (PE / EA=3 / 1) to obtain the target compound (3.30 g, yield: 80%) as a white solid.
[0098] Step 2: Preparation of 2-aminobenzo[d]thiazole-6-carboxylic acid methyl ester
[0099]
[0100] Under the p...
Embodiment 3
[0109] Example 3: 6-(3-((5-cyclopropyl-3-(2-(trifluoromethyl)phenyl)isoxazol-4-yl)methoxy)-1-oxa-8 -Azaspiro[4.5]decane-8-yl) nicotinic acid
[0110] The synthesis steps are as follows:
[0111]
[0112] Step 1: 6-(3-((5-cyclopropyl-3-(2-(trifluoromethyl)phenyl)isoxazol-4-yl)methoxy)-1-oxa-8- Preparation of azaspiro[4.5]decane-8-yl) nicotinic acid ethyl ester
[0113]
[0114] Will K 2 CO 3 (124mg, 0.9mmol), 3-((5-cyclopropyl-3-(2-(trifluoromethyl)phenyl)isoxazol-4-yl)methoxy)-1-oxa-8 -Azaspiro[4.5]decane (211 mg, 0.5 mmol) and ethyl 6-fluoronicotinate (118 mg, 0.7 mmol) were dispersed in DMF (6 mL), and reacted at 100°C under nitrogen protection for 3.5 hours. After cooling, the reaction system was poured into water, EA was added for extraction three times, the organic layers were combined, washed with water and saturated brine, anhydrous Na 2 SO 4 The crude product obtained after drying and concentration was separated and purified by a thin-layer preparation plate (PE / EA=3 / 1) to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com