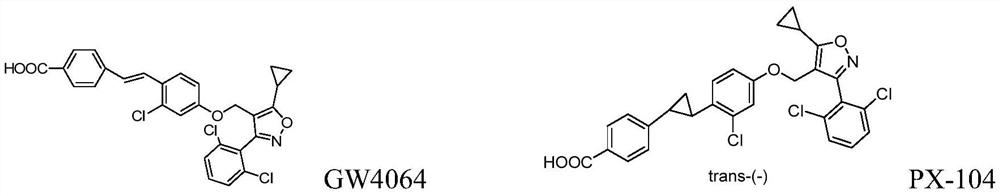
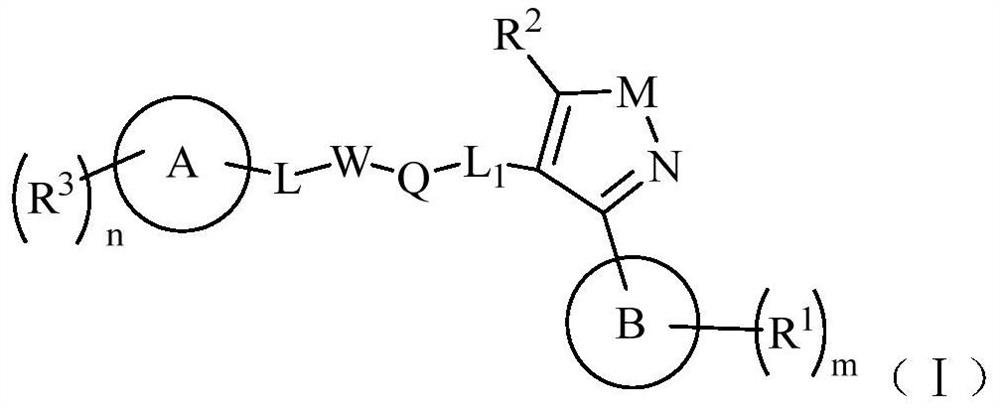
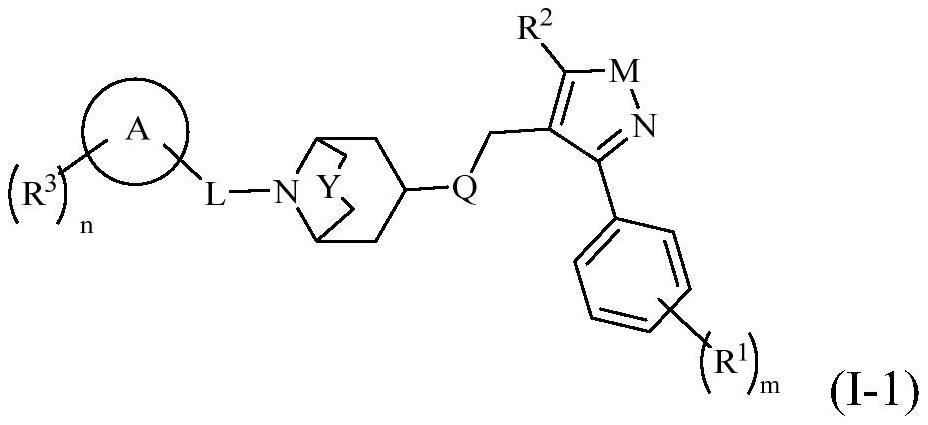
fxr receptor agonist
A technology selected from, alkyl, applied in the field of FXR receptor agonists, can solve problems such as unknown structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0212] 1. Preparation of Intermediate 1
[0213] Intermediate 1 was purchased or prepared.
[0214] 2. Preparation of Intermediate 2
[0215] Intermediate 2 was purchased or prepared.
[0216] 3. Preparation of Intermediate 3
[0217] Dissolve intermediate 1 in an organic solvent containing a catalyst (such as tetrahydrofuran, etc.), add an alkaline reagent (such as potassium tert-butoxide) under ice-cooling, and maintain the reaction at 0°C, then add intermediate 2, and rise to 10-35°C React for 8-16 hours, remove the solvent, and purify (purification method is preferably: preparative high performance liquid chromatography, silica gel column chromatography, etc.) to obtain intermediate 3.
[0218] 4. Preparation of Intermediate 4
[0219] Dissolve intermediate 3 in an organic solvent (such as ethanol, etc.) at a low temperature (such as 0° C.), add an acidic solution (such as an ethanol solution containing HCl, etc.), and react for 5-30 hours. After the reaction is comple...
experiment example 1
[0234] Experimental Example 1: The effect of the compounds of the present invention on the relative expression of BSEP mRNA in HepG2 cells
[0235] (1) Test substance: the compound of the present invention, its chemical name and preparation method are shown in the preparation examples of each compound.
[0236] PBS stands for phosphate buffered saline.
[0237] Control drug: PX-104, see background technology for specific structure.
[0238] (2) Experimental method:
[0239] ①Laying cells, adding compounds and collecting cells
[0240] Use trypsin to digest and collect the cells, and measure the cell concentration; according to the counting results, resuspend the cells to a density of 7.5e5cell / mL; inoculate 2 mL of cells in each well of a 6-well cell culture plate; place the culture plate in an incubator, at 37°C, 5%CO 2 Conditioned for 24 hours.
[0241] Dilute the compound to be tested to 0.3 and 3 mM with DMSO; take 5 μL of the stock solution diluted in the previous s...
experiment example 2
[0265] Experimental example 2: Experiment on the metabolic stability of liver microsomes of compounds of the present invention
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com