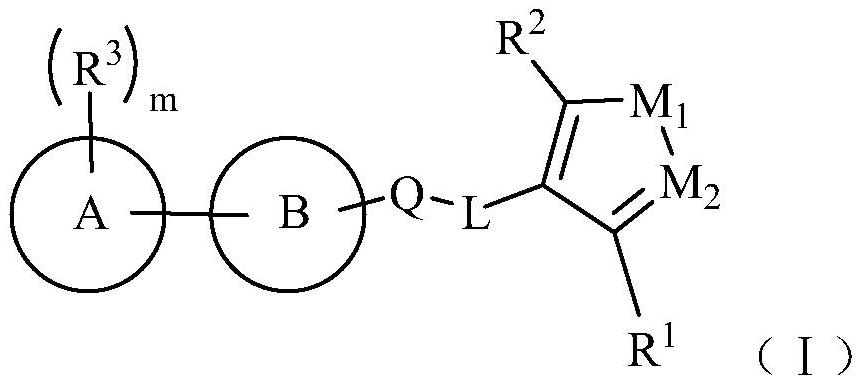
fxr receptor agonist
A compound, selected technology, used in anti-inflammatory agents, non-central analgesics, metabolic diseases, etc., can solve problems such as unknown structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0126] 1. Preparation of Intermediate 1
[0127] Dissolve starting material 1 (self-made or purchased), alkaline solution and phase transfer catalyst in an organic solvent, add starting material 2 (self-made or purchased), and react at 25°C-50°C. After the reaction was completed, cooled, the solvent was removed from the reaction liquid under reduced pressure, and purified by silica gel column chromatography to obtain intermediate 1. The alkaline solution is selected from sodium hydrogen, potassium tert-butoxide, sodium tert-butoxide, sodium hydroxide, potassium hydroxide, sodium methylate, sodium ethylate, potassium iodide, sodium iodide, etc., preferably potassium tert-butoxide, potassium iodide, iodine Sodium chloride; the organic solvent is selected from tetrahydrofuran, DMF, toluene, acetonitrile, etc., preferably tetrahydrofuran; the phase transfer catalyst is selected from 18-crown-6, tetrabutylammonium iodide, etc., preferably 18-crown-6.
[0128] 2. Preparation of Int...
experiment example 1
[0145] Experimental Example 1: The effect of the compounds of the present invention on the relative expression of BSEP mRNA in HepG2 cells
[0146] Test product: the compound of the present invention, its chemical name and preparation method are shown in the preparation examples of each compound.
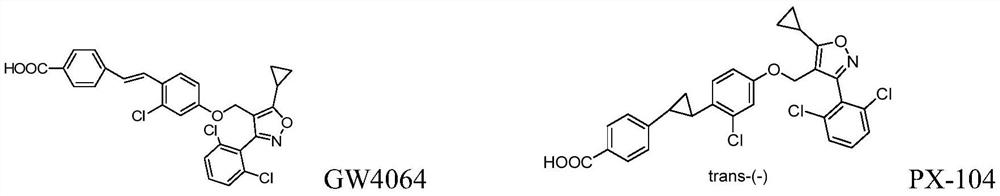
[0147] Reference substance: compound PX-104, prepared according to the method of the prior art, its structure is shown in the background art.
[0148] Reagents: PBS: Phosphate buffer saline.
[0149] experimental method:
[0150] 1. Plating cells, adding compounds and collecting cells
[0151] Use trypsin to digest and collect the cells, and measure the cell concentration; according to the counting results, resuspend the cells to a density of 7.5e5cell / mL; inoculate 2 mL of cells in each well of a 6-well cell culture plate; place the culture plate in an incubator, at 37°C, 5%CO 2 Conditioned for 24 hours.
[0152] Dilute the compound to 3,0.3mM with DMSO; take 5ul of the stock s...
experiment example 2
[0181] Experimental example 2: The metabolic stability experiment of liver microsomes of the compound of the present invention in different species
[0182] Test product: compound of the present invention, self-made, its chemical name and preparation method are shown in the preparation examples of each compound.
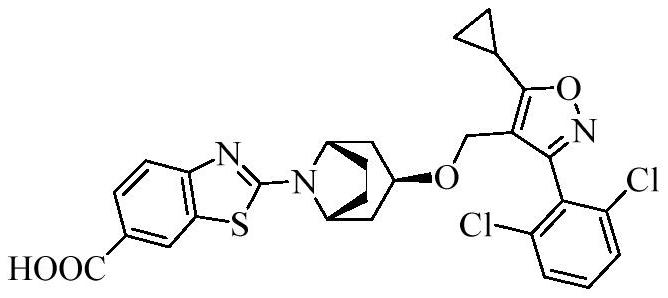
[0183] Reference substance: compound 30-70, prepared according to the method of the prior art, and its structure is shown in the background art.
[0184] Experimental Materials:
[0185] The mixed liver microsomes of SD rats, Beagle dogs, and CD-1 mice were purchased from XenoTech, the batch numbers are: 1410271 (SD rats), 1310086 (Beagle dogs), 1510043 (CD-1 mice), liver microsomes The protein concentration is 20mg·mL -1 .
[0186] The mixed liver microsomes of Cyno monkeys were purchased from Reid Liver Disease Research Center (Shanghai Co., Ltd.), the batch number is: NMZC, and the concentration of liver microsomal protein is 20 mg·mL -1 .
[0187] Human mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com