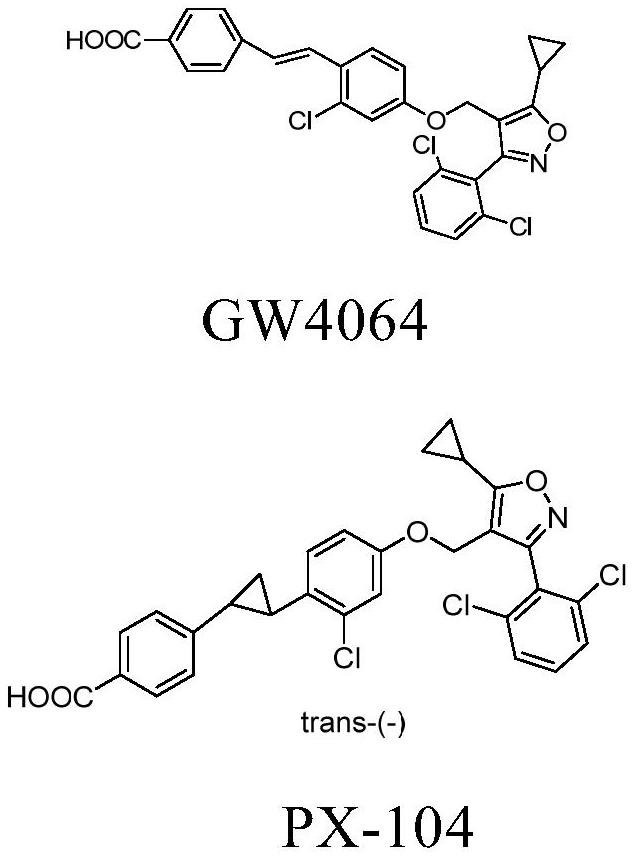
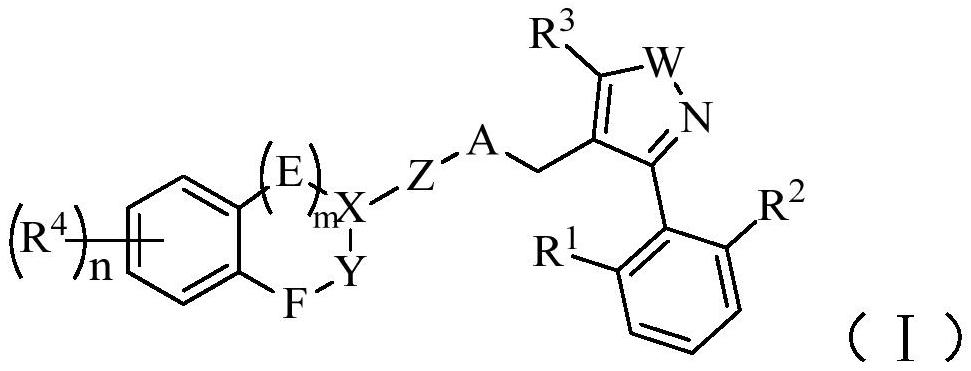
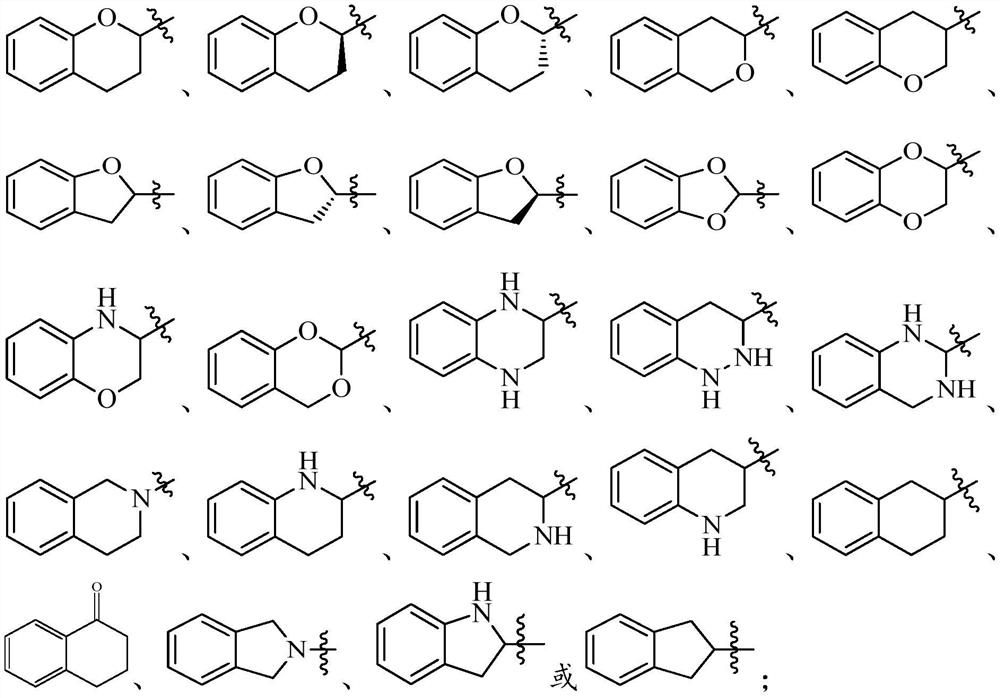
fxr receptor agonist
A technology selected from compounds, applied in anti-inflammatory agents, non-central analgesics, metabolic diseases, etc., can solve problems such as low bioavailability and instability to light
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0340] Preparation Example 1: Preparation of (E)-2,6-dichlorobenzaldehyde oxime
[0341]
[0342] Dissolve 2,6-dichlorobenzaldehyde (25g, 0.14mol) in ethanol (200mL), slowly add hydroxylamine hydrochloride (11g, 0.16mol) in batches, after the addition is complete, add NaOH solution (6.4g, 0.16mol, in 100mL water), heated to 90°C for 24 hours. After the reaction was completed, the solvent was removed from the reaction solution under reduced pressure, and the solid was washed with water (200 mL) and dried to obtain 25.9 g of the title compound with a yield of 97%.
preparation example 2
[0343] Preparation Example 2: Preparation of (Z)-2,6-dichloro-N-hydroxyiminobenzyl chloride
[0344]
[0345] Dissolve (E)-2,6-dichlorobenzaldehyde oxime (25.9g, 0.136mol) into DMF (300mL), and slowly add N-chlorosuccinimide (18.2g, 0.136mol) in batches, After the addition, the mixture was stirred at 25°C for one hour, and the reaction solution was poured into water (500 mL). Extracted with ethyl acetate (500 mL), washed the organic phase with water (200 mL) and saturated sodium chloride solution (200 mL), dried over anhydrous sodium sulfate, and removed the solvent to obtain 28 g of the title compound. The crude product was carried on to the next step without purification.
preparation example 3
[0346] Preparation Example 3: Preparation of ethyl 5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazole-4-carboxylate
[0347]
[0348] Dissolve (Z)-2,6-dichloro-N-hydroxyiminobenzyl chloride (28g, 0.125mol) in triethylamine (100mL), add ethyl 3-cyclopropyl-3-oxopropionate (19.5g, 0.125mol), react at 25°C for 12 hours. After the reaction was completed, the solvent was removed under reduced pressure and separated by column chromatography (PE:EA=10:1) to obtain 24.4 g of the title compound, with a two-step yield of 55.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com