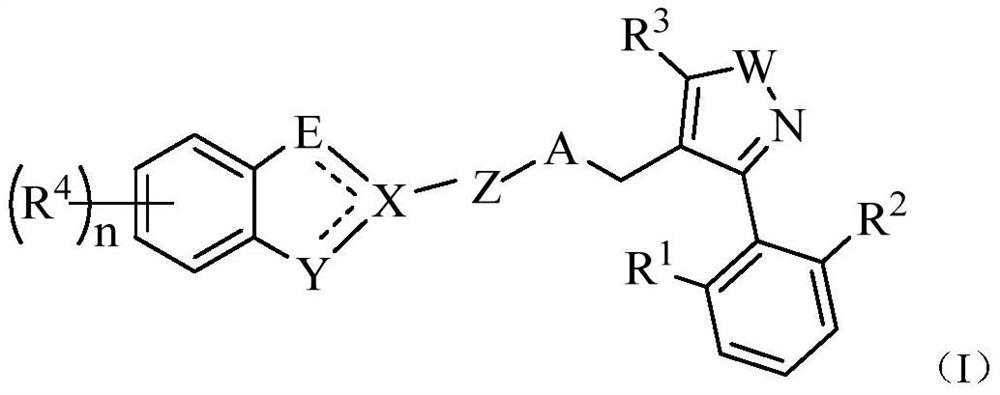
fxr receptor agonist
A stereoisomer and selection technology, applied in anti-inflammatory agents, non-central analgesics, metabolic diseases, etc., can solve problems such as unknown structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0107] The present invention also provides the preparation method of the compound of formula (I), which includes but not limited to the following process route:
[0108] The definitions represented by each abbreviation are as follows:
[0109] THF: tetrahydrofuran; EA: ethyl acetate; PE: petroleum ether; MeOH: methanol.
[0110]
[0111] R 1 , R 2 , R 3 , R 4 , W, A, Z, E, X, Y, n are as described above, and a' represents a fluorine atom, a chlorine atom, a bromine atom and an iodine atom.
[0112] The specific exemplary steps are as follows:
[0113] 1. Raw material 1: purchased or prepared.
[0114] 2. Raw material 2: purchased or prepared.
[0115] 3. Preparation of Intermediate 1
[0116] After acidifying raw material 2 with acid, it was dissolved in an organic solvent with raw material 1, reacted at 100°C for 12 hours, concentrated, and purified by silica gel column chromatography (petroleum ether:ethyl acetate=3:1) to obtain intermediate 1. Wherein the acid i...
experiment example 1
[0130] Experimental example 1: Effects of the compounds of the present invention on the relative expression of BSEP mRNA in HepG2 cells and human hepatocytes
[0131] (1) Test substance: the compound of the present invention, its chemical name and preparation method are shown in the preparation examples of each compound.
[0132] PBS: Phosphate buffered saline.
[0133] (2) Experimental method:
[0134] 1. Plating cells, adding compounds and collecting cells
[0135] Use trypsin to digest and collect the cells, and measure the cell concentration; according to the counting results, resuspend the cells to a density of 7.5e5cell / mL; inoculate 2 mL of cells in each well of a 6-well cell culture plate; place the culture plate in an incubator, at 37°C, 5%CO 2Conditioned for 24 hours.
[0136] Dilute the compound to 3,0.3mM with DMSO; take 5ul of the stock solution diluted in the previous step and add it to 5ml of the culture medium. The concentrations of the obtained working s...
Embodiment 1
[0160] Example 1: 2-((2R,4r,6S)-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl)methoxy)- 2,6-Dimethylpiperidin-1-yl)benzo[d]thiazole-6-carboxylic acid
[0161]
[0162] 1. Preparation of (2R,6S)-1-benzyl-2,6-dimethylpiperidin-4-one
[0163]
[0164] 3-Carbonyl glutaric acid (40.0g, 0.28mol) and 40% acetaldehyde (60.5g, 1.37mol) were stirred at 25°C for 10min, then slowly added benzylamine (30mL, 0.28mol), and the reaction solution was Stir at ℃ for 78 h, adjust the pH to 2 with 1N hydrochloric acid, and then stir for 1 h, then adjust the pH to 7 with saturated sodium bicarbonate solution, extract with dichloromethane, dry over anhydrous sodium sulfate, concentrate, and perform silica gel column chromatography (petroleum ether: acetic acid Ethyl ester=9:1) to obtain the cis-title compound (2 g, yield: 3.4%).
[0165] 2. Preparation of (2R, 6S)-2,6-dimethylpiperidin-4-one
[0166]
[0167] Add (2R,6S)-1-benzyl-2,6-dimethylpiperidin-4-one (2g, 9.2mol) and 20% p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com