Nisoldipine controlled release tablet and preparation method thereof
A technology for nisoldipine and controlled-release tablets, which is applied in pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as low production efficiency, complicated three-layer tablet process, and large quality differences, and achieve production The effect of cost reduction, ease of scale-up production, and improvement of production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Dissolution test of nisoldipine obtained by various treatment methods
[0048] Nisoldipine is almost insoluble in water, and its solubility in water is determined to be only 0.000036 mg / ml.
[0049] 1. Treat nisoldipine as follows:
[0050] (1) non-micronized conventional preparation powder;
[0051] (2) carry out micronization treatment with nisoldipine by conventional method;
[0052] (3) according to the inventive method described above, carry out micronization treatment after nisoldipine and sodium lauryl sulfate are uniformly dispersed together;
[0053] (4) according to the inventive method described above, first carry out micronization treatment with nisoldipine, then handle with sodium lauryl sulfate solution;
[0054] (5) The nisoldipine and sodium lauryl sulfate are respectively micronized by a conventional method.
[0055] 2. The nisoldipine that is not micronized, the nisoldipine that is micronized separately, and the micronized nisoldipine tha...
Embodiment 2
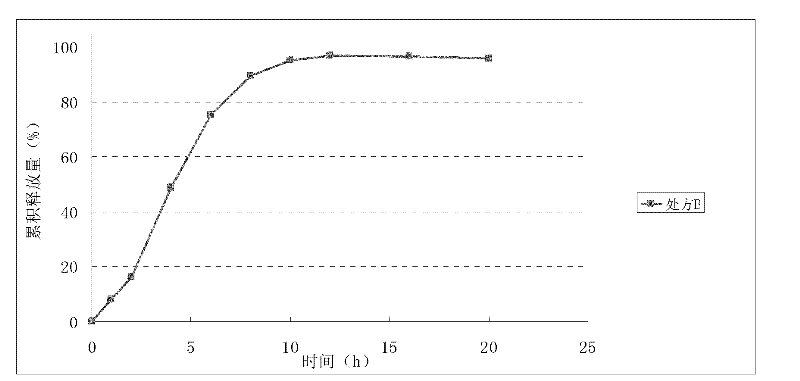
[0067] The effect of embodiment 2 micronization on the release rate of nisoldipine
[0068] After solving the dissolution rate of nisoldipine, the key is to solve the release mode and release rate of nisoldipine controlled-release tablets. Patents US20080063711, US20080057123, US20080221174, CN200780034924.1, WO2008025532 improved the bioavailability by changing the release site of nisoldipine compared with patents US5422123 and US5626874, because the bioavailability of nisoldipine was only about 5%. There is intestinal wall metabolism in the intestinal tract, and it is less metabolized in the lower part of the intestinal tract than in the upper part of the intestinal tract. The existing patent controls the release area of the tablet during initial release through the three-layer tablet technology and uses pH-dependent excipients to control the release of nisoldipine in various parts of the digestive tract.
[0069] The inventors used a single-layer tablet containing pH-dep...
Embodiment 3
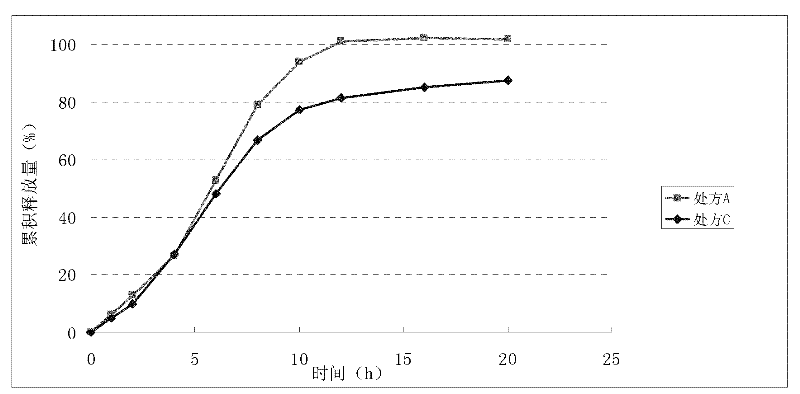
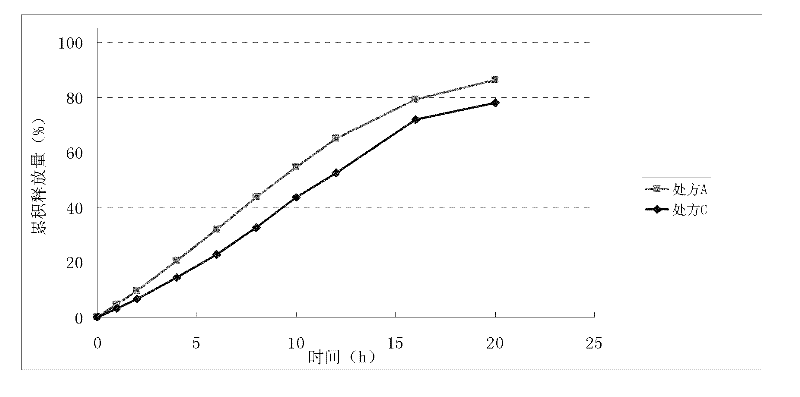
[0114] Example 3 Release comparison of different doses of nisoldipine formulations in buffered saline solutions with different pH 6.8 values
[0115] Prescription D (per 1000 tablets):
[0116] Nisoldipine 8.5g 3.7%
[0117] Sodium Lauryl Sulfate 8.5g 3.7%
[0118] Acrylic resin 10g 4.4%
[0119] Hypromellose 95g 41.5%
[0120] Lactose 103g 45.0%
[0121] Micronized silica gel 2.5g 1.1%
[0122] Magnesium Stearate 1.25g 0.6%
[0123] Total solids 228.75 100.0%
[0124] Preparation process: same as prescription A.
[0125] Prescription E (per 1000 tablets):
[0126] Nisoldipine 25.5g 9.5%
[0127] Sodium Lauryl Sulfate 31.875g 11.8%
[0128] Acrylic resin 10g 3.7%
[0129] Hypromellose 95g 35.3%
[0130] Lactose 103g 38.3%
[0131] Micropowder silica gel 2.5g 0.9%
[0132] Magnesium Stearate 1.25g 0.5%
[0133] Total solids 269.125 100%
[0134] Preparation process: same as prescription A.
[0135] Prescription F (per 1000 tablets):
[0136] Nisoldipine 34g 11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com