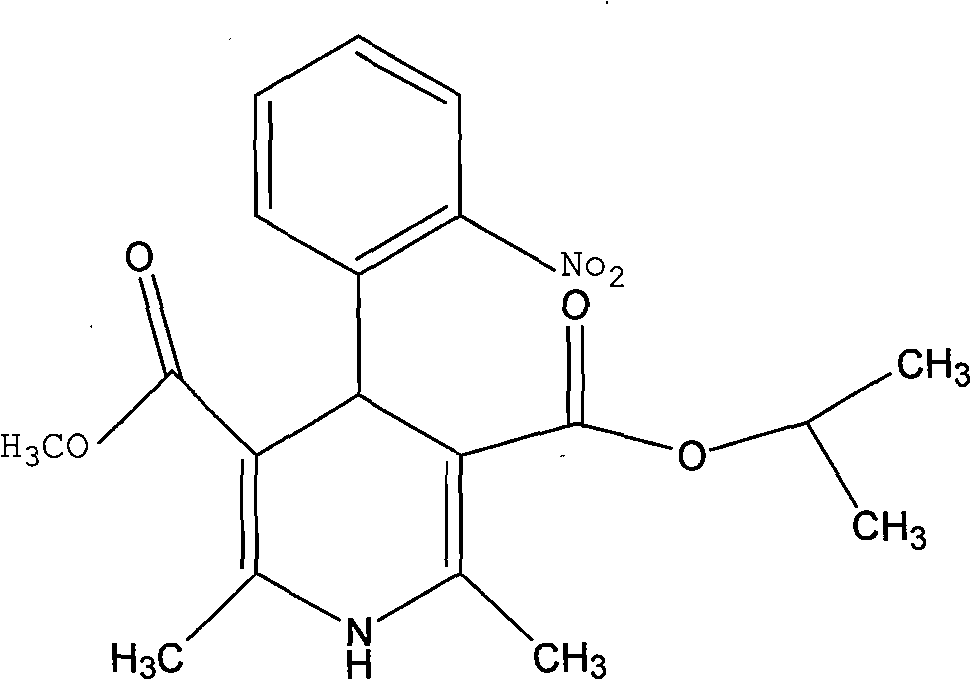
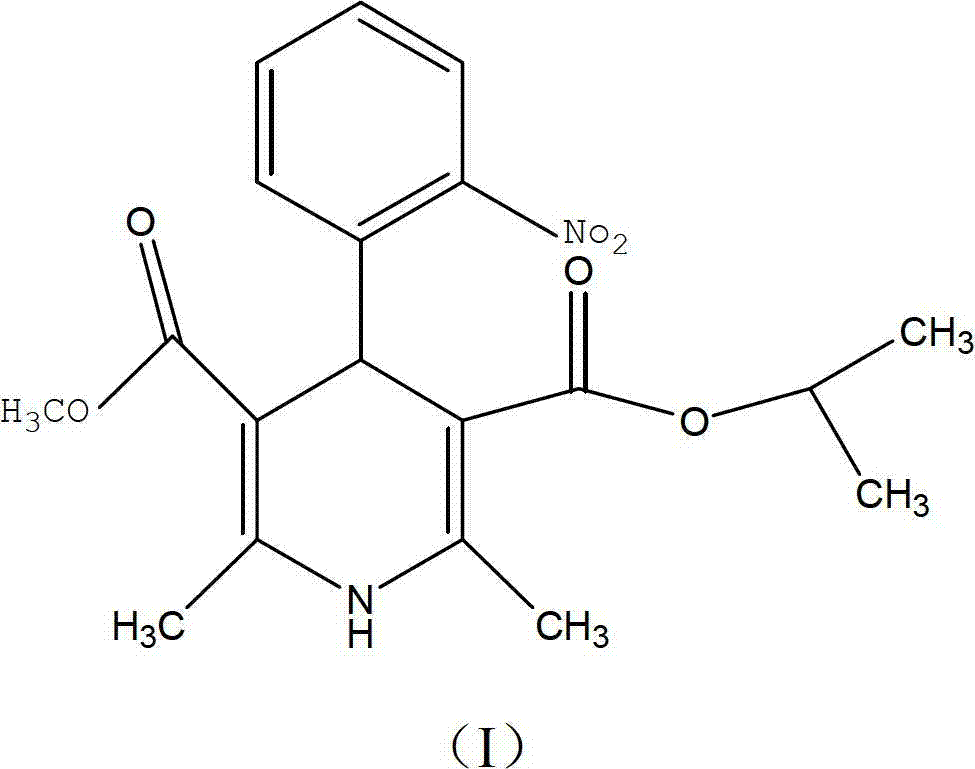
Nisoldipine compound and novel preparation method thereof
A technology for nisoldipine and a compound, which is applied in the field of nisoldipine compounds and new preparation methods thereof, can solve the problems of unsatisfactory drug purity, no public purification method, and reduced content of drug active ingredients, and achieves the improvement of clinical adverse reactions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The refining of embodiment 1 nisoldipine
[0053] Dissolve 10 g of the crude product of nisoldipine with a purity of 96.52% in 40 ml of acetone, stir to make it completely dissolved, then add 0.2 g of activated carbon, stir and adsorb at 40°C for 15 minutes, filter for decarburization, and collect the filtrate; after concentrating under reduced pressure at 60°C Then add 10g of neutral alumina and stir evenly, add to the upper end of the prepared chromatographic column after evaporating the solvent, and then carry out separation and purification with a preparative chromatographic column, wherein the mobile phase used in the chromatographic column is acetone with a volume ratio of 30:50:20 : Mixed solution of ethanol: water, flow rate 1ml / min, stationary phase filler is ICN neutral alumina with a particle size of 18-32μm and a pore size of 6nm, column temperature is room temperature, wavelength 237nm, column pressure 1.0MPa, collect and elute liquid, and then concentrated...
Embodiment 2
[0057] The refining of embodiment 2 nisoldipine
[0058] Dissolve 10 g of nisoldipine crude product with a purity of 96.52% in 100 ml of acetone, stir to make it completely dissolved, then add 0.1 g of activated carbon, stir and adsorb at 50°C for 10 minutes, filter for decarburization, and collect the filtrate; after concentrating under reduced pressure at 50°C Then add 10g of neutral alumina and stir evenly, evaporate the solvent and add to the upper end of the prepared chromatographic column, then use the preparative chromatographic column to separate and purify the filtrate, wherein the mobile phase used in the chromatographic column is 30:50:20 by volume Acetone: ethanol: mixed solution of water, flow rate 2ml / min, stationary phase filler is particle diameter 50-200μm, pore diameter 6nm Baker column chromatography special neutral alumina, column temperature is room temperature, wavelength 237nm, column pressure 2.5MPa , collect the eluate, and then concentrate it under re...
Embodiment 3
[0062] The refining of embodiment 3 nisoldipine
[0063] Dissolve 10 g of the crude product of nisoldipine with a purity of 96.52% in 60 ml of acetone, stir to make it completely dissolved, then add 0.3 g of activated carbon, stir and adsorb at 45°C for 10 minutes, filter for decarburization, and collect the filtrate; after concentrating under reduced pressure at 55°C Then add 15g of neutral alumina and stir evenly, evaporate the solvent and add to the upper end of the prepared chromatographic column, then use the preparative chromatographic column to separate and purify the filtrate, wherein the mobile phase used in the chromatographic column is 30:50:20 by volume Acetone: ethanol: mixed solution of water, flow rate 1.7ml / min, stationary phase filler is ICN neutral alumina with a particle size of 18-32 μm and a pore size of 6 nm, the column temperature is room temperature, the column pressure is 2.0 MPa, and the wavelength is 237 nm. Collect the eluent, then concentrate it un...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com