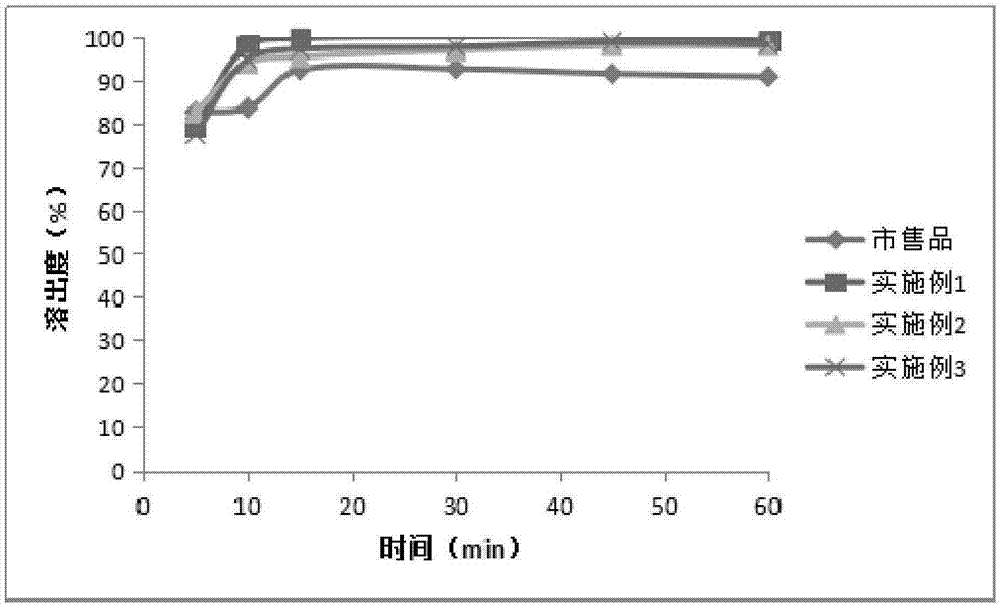
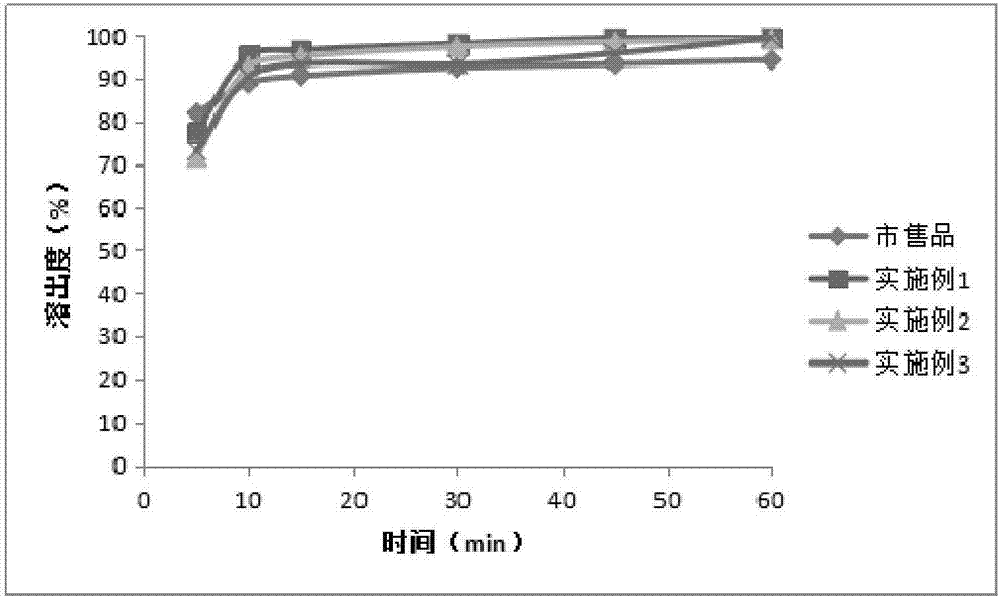
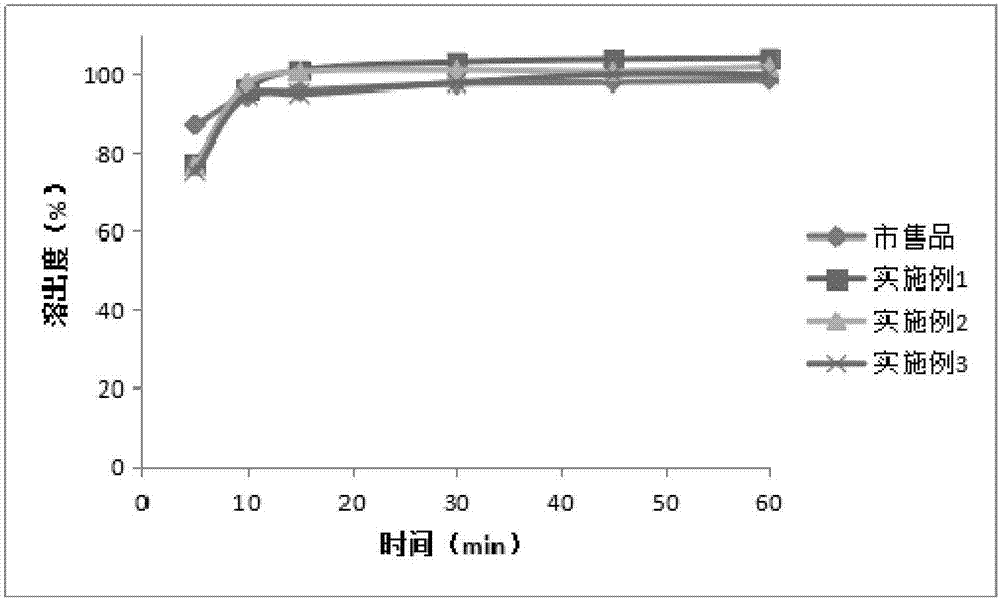
Azithromycin tablet and preparation method thereof
A technology of azithromycin tablets, azithromycin, applied in the field of medicine to achieve the effect of good quality, good compressibility and high reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 azithromycin sheet of the present invention
[0031] 1. Raw and auxiliary materials: 250g of azithromycin, 30g of lactose, 100g of anhydrous calcium hydrogen phosphate, 4.59 parts of hypromellose, 4g of sodium lauryl sulfate, 26g of croscarmellose sodium, 4g of silicon dioxide, hard Magnesium fatty acid 1g, Opadry coating powder 40g
[0032] 2. Preparation method:
[0033] 1) Weigh 250g of azithromycin, 30g of lactose, 100g of anhydrous calcium hydrogen phosphate and mix well;
[0034] 2) E30 hypromellose was prepared into a solution with a mass fraction of 4.59%, and 100ml was added to make granules;
[0035] 3) drying at 60°C;
[0036] 4) passing the obtained dry granules through a 20-mesh sieve, and sizing;
[0037] 5) Add 4 g of sodium lauryl sulfate, 26 g of croscarmellose sodium, 4 g of silicon dioxide and 1 g of magnesium stearate through a 100-mesh sieve according to weight, and mix evenly again;
[0038] 6) tablet forming to...
Embodiment 2
[0041] The preparation of embodiment 2 azithromycin tablets of the present invention
[0042] 1. Raw materials: 250g of azithromycin, 35g of lactose, 80g of anhydrous calcium hydrogen phosphate, 3g of hypromellose, 3g of sodium lauryl sulfate, 20g of croscarmellose sodium, 3g of silicon dioxide, stearin Magnesium acid 1g, Opadry coating powder 40g.
[0043] 2. Preparation method:
[0044] 1) Weigh 250g of azithromycin, 35g of lactose, and 80g of anhydrous calcium hydrogen phosphate and mix well;
[0045] 2) Prepare E30 hypromellose into a solution with a mass fraction of 3%, take 100ml and add it to make granules;
[0046] 3) drying at 60°C;
[0047] 4) passing the obtained dry granules through a 20-mesh sieve, and sizing;
[0048] 5) Add 3 g of sodium lauryl sulfate, 20 g of croscarmellose sodium, 3 g of silicon dioxide and 1 g of magnesium stearate passed through a 100-mesh sieve according to weight, and mix evenly again;
[0049] 6) tablet forming to obtain azithromyci...
Embodiment 3
[0052] The preparation of embodiment 3 azithromycin tablets of the present invention
[0053] 1. Raw materials: Azithromycin 250g, lactose 40g, anhydrous calcium hydrogen phosphate 100g, hypromellose 8g, sodium lauryl sulfate 8g, croscarmellose sodium 40g, silicon dioxide 8g, stearin Magnesium acid 1g, Opadry coating powder 40g.
[0054] 2. Preparation method:
[0055] 1) Weigh 250g of azithromycin, 40g of lactose, 100g of anhydrous calcium hydrogen phosphate and mix well;
[0056] 2) Prepare E30 hypromellose into a solution with a mass fraction of 8%, and add 100ml to make granules;
[0057] 3) drying at 60°C;
[0058] 4) passing the obtained dry granules through a 20-mesh sieve, and sizing;
[0059] 5) Add 8 g of sodium lauryl sulfate, 40 g of croscarmellose sodium, 8 g of silicon dioxide and 1 g of magnesium stearate through a 100-mesh sieve according to weight, and mix again;
[0060] 6) tablet forming to obtain azithromycin tablets;
[0061] 7) Weigh 40g of white Opad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com