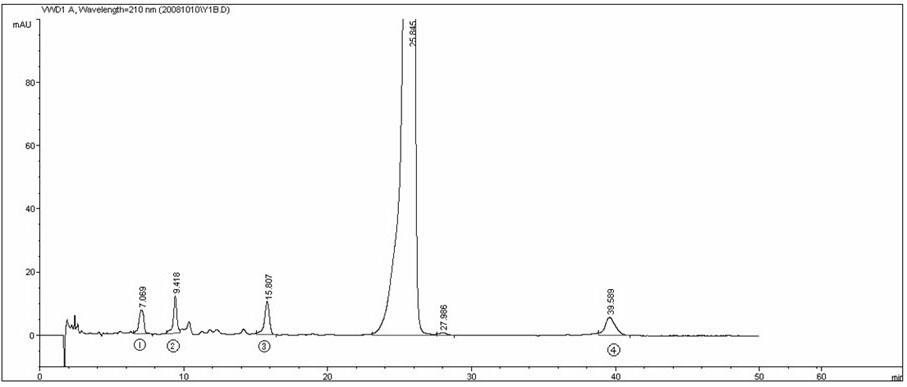
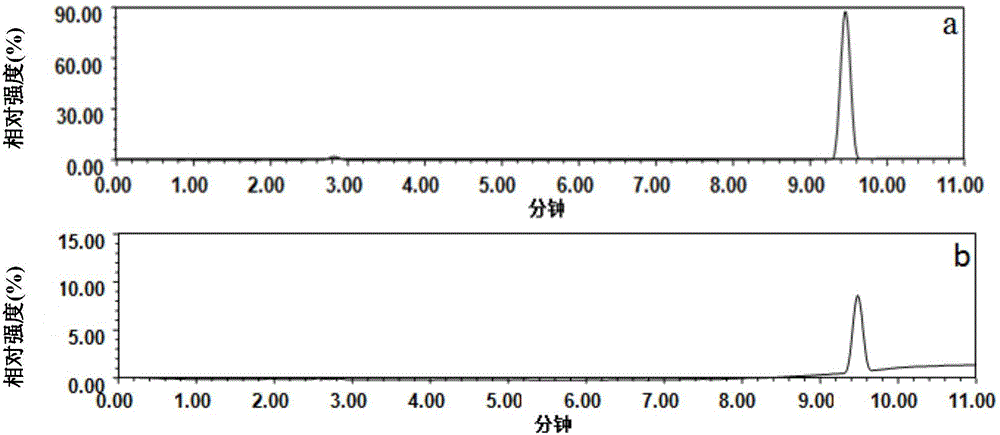
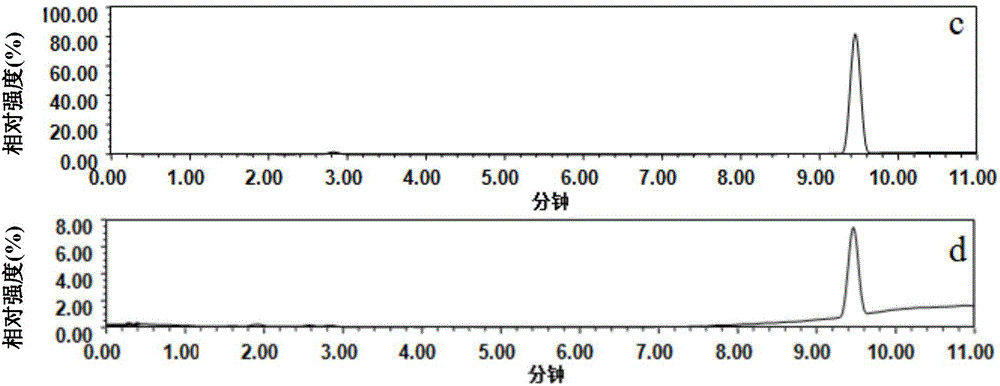
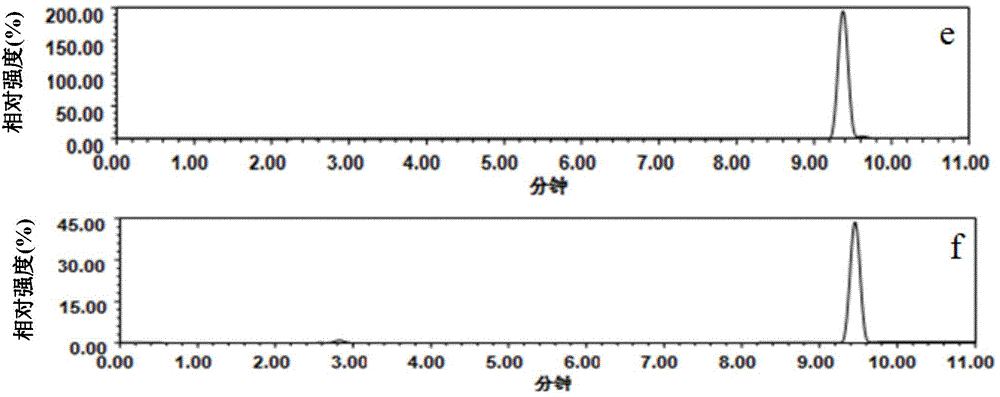
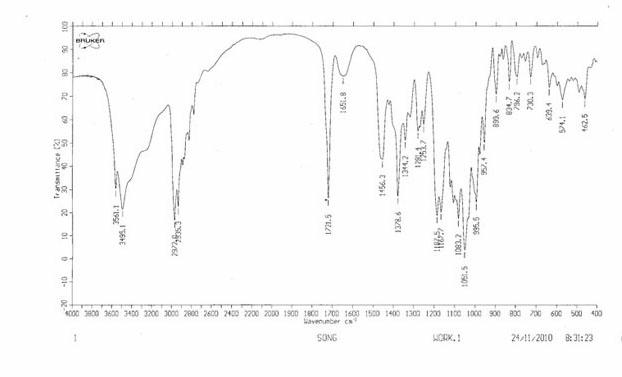
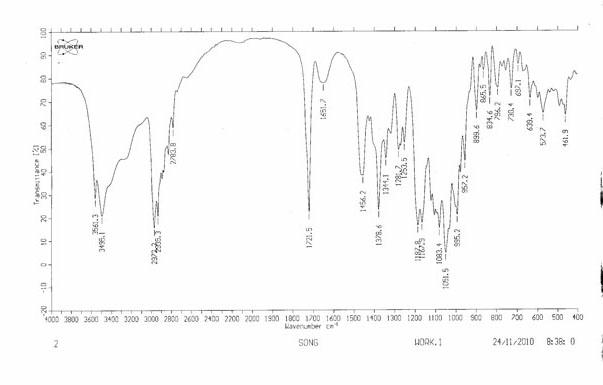
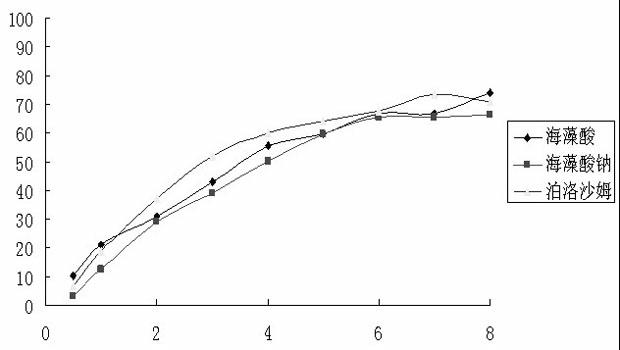
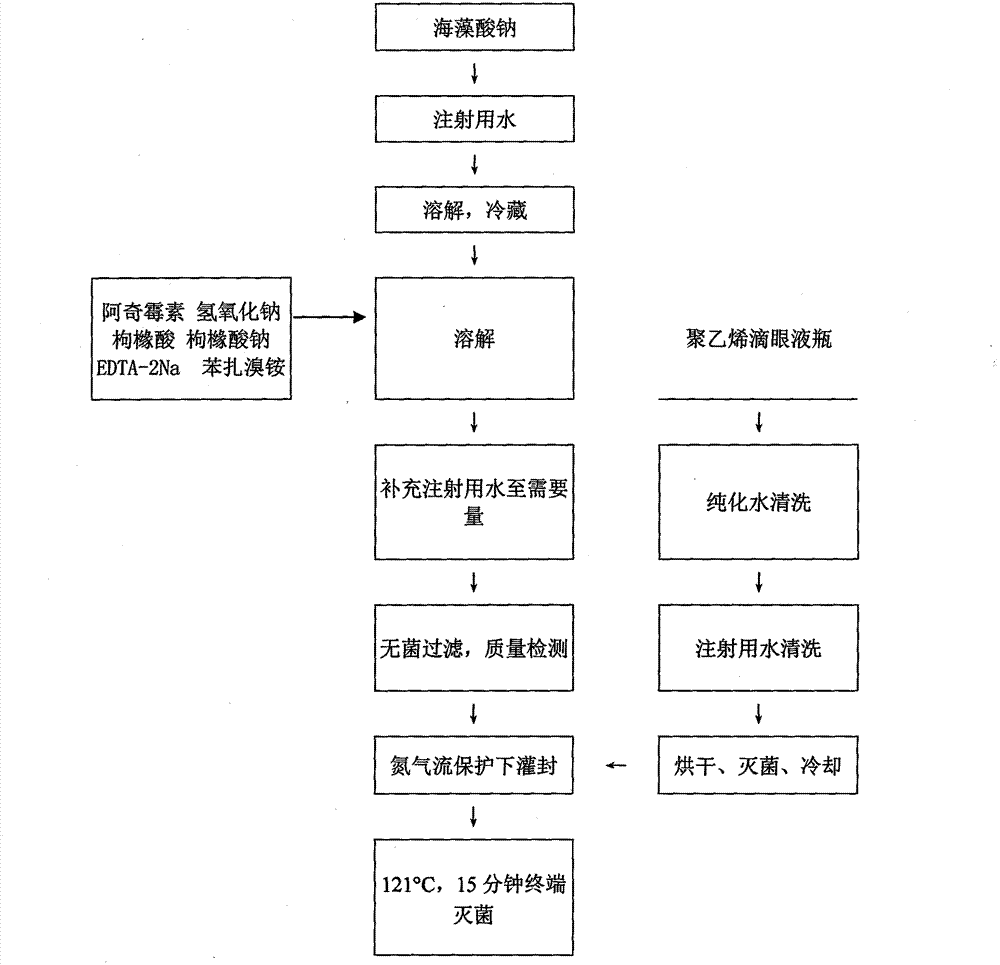
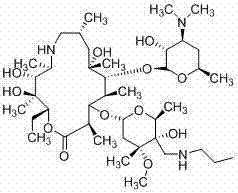
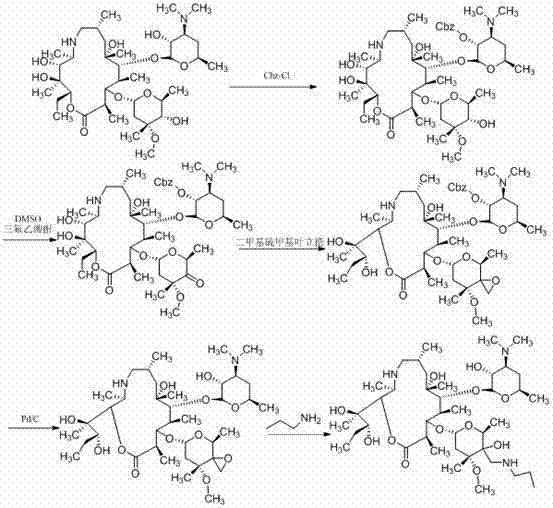
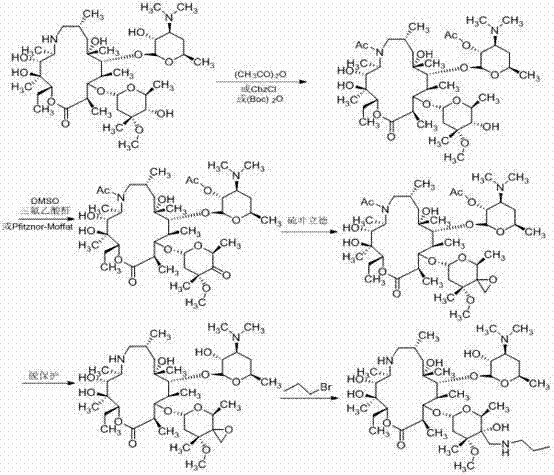
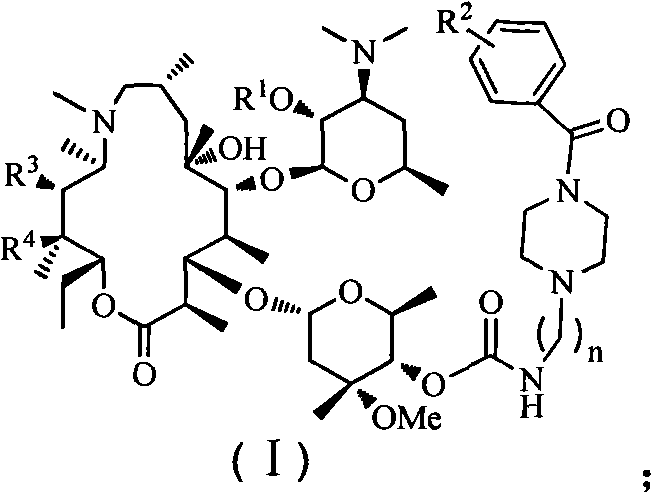
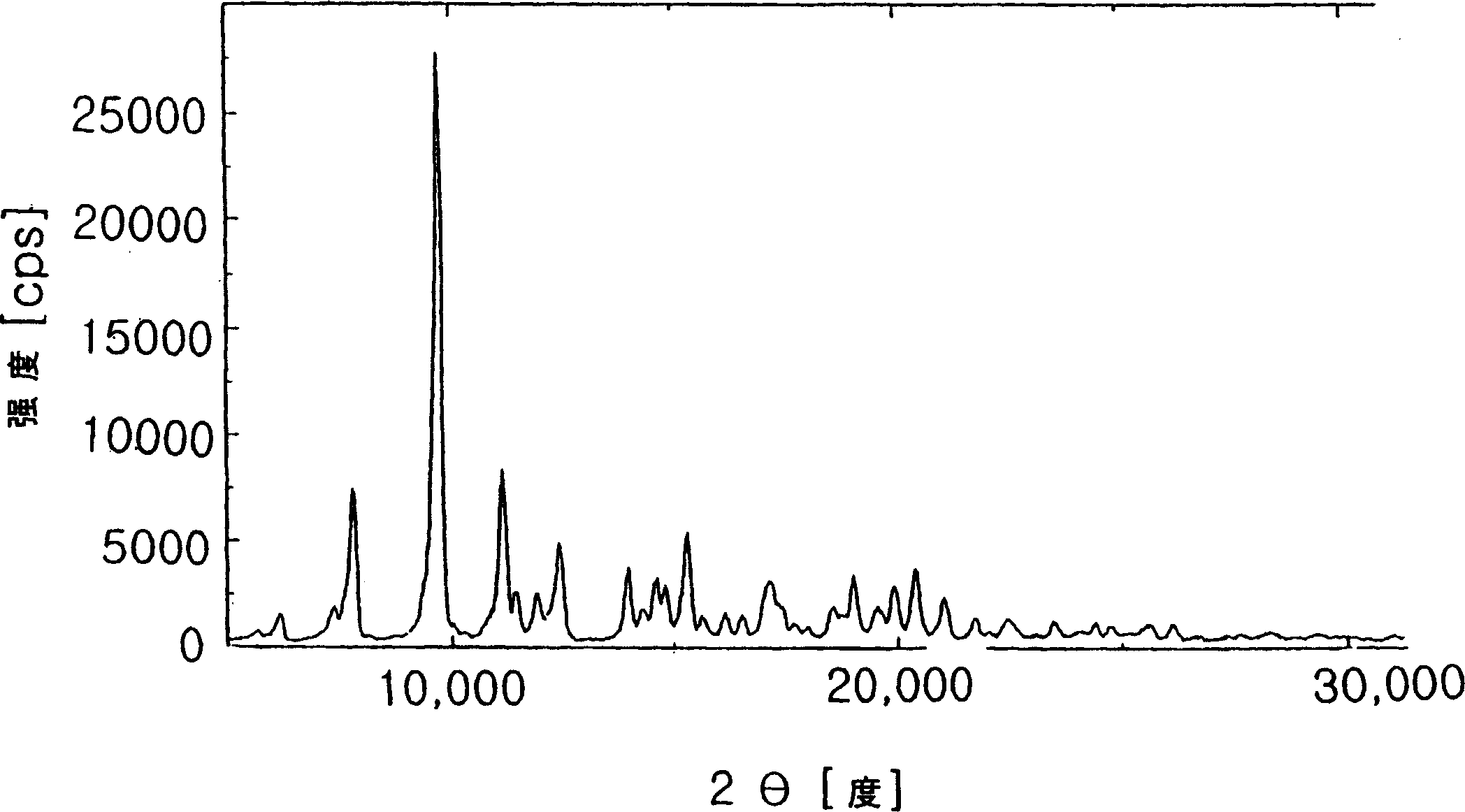
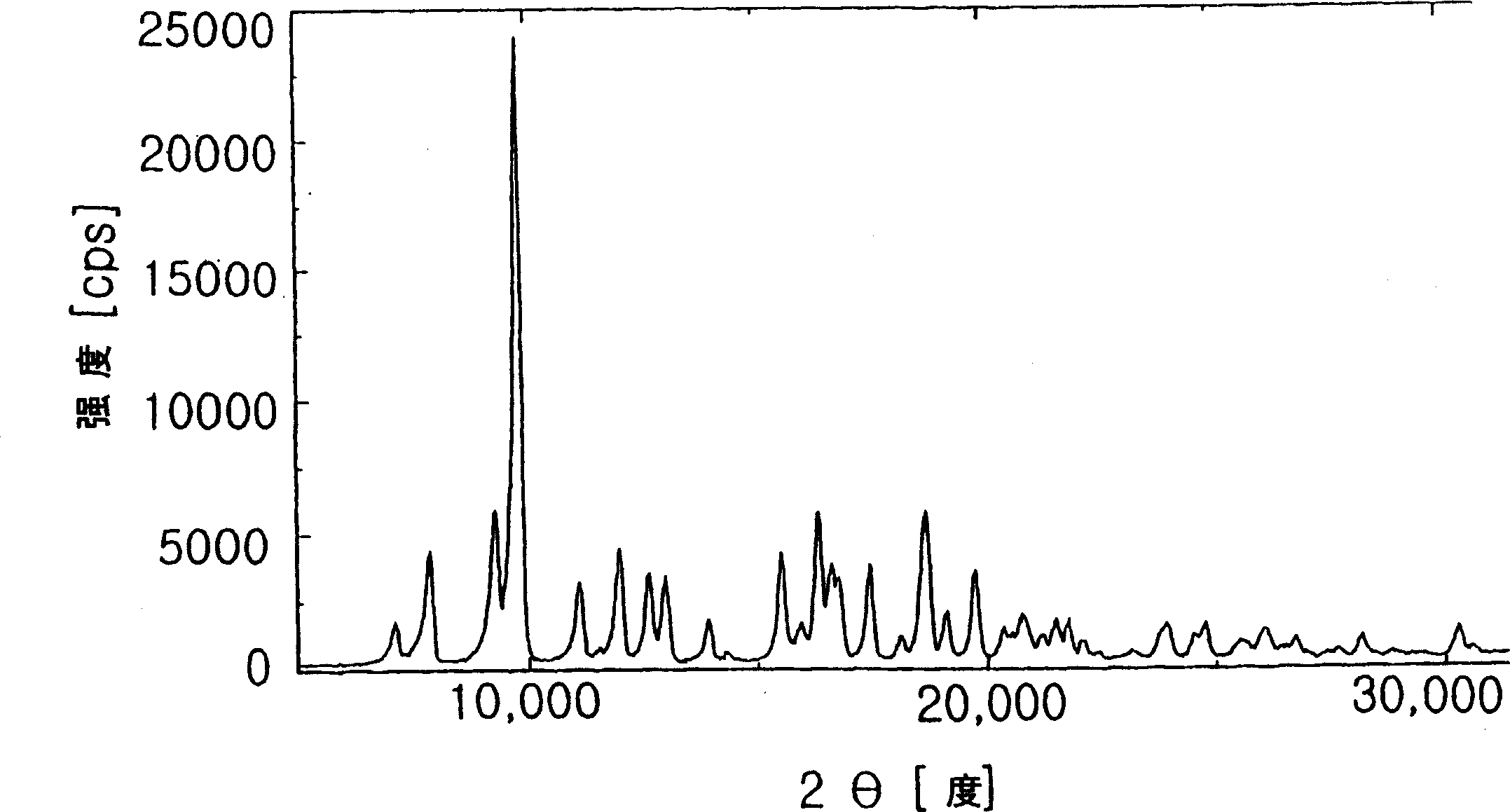
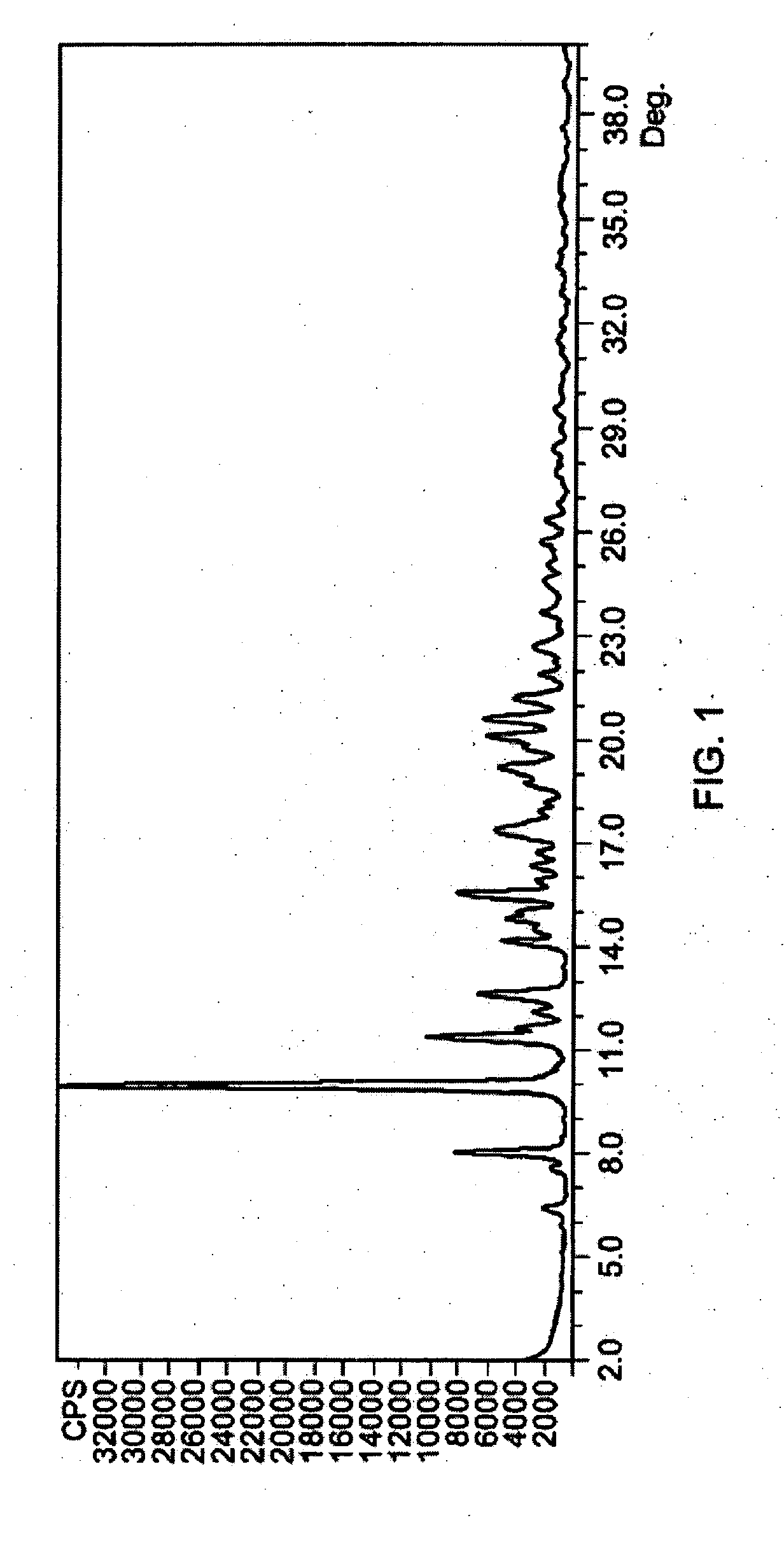
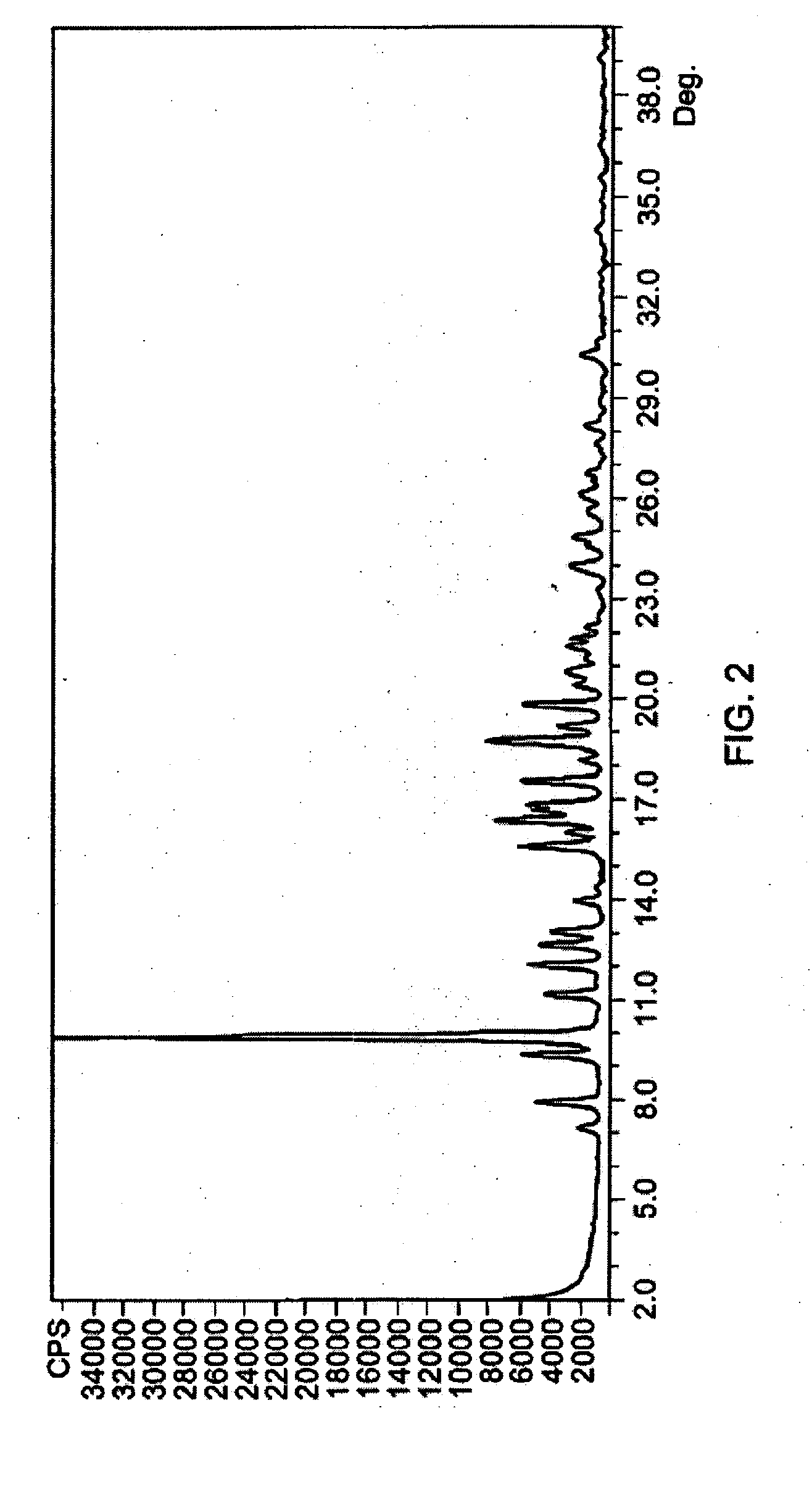
Patents
Literature
151 results about "Azythromycin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
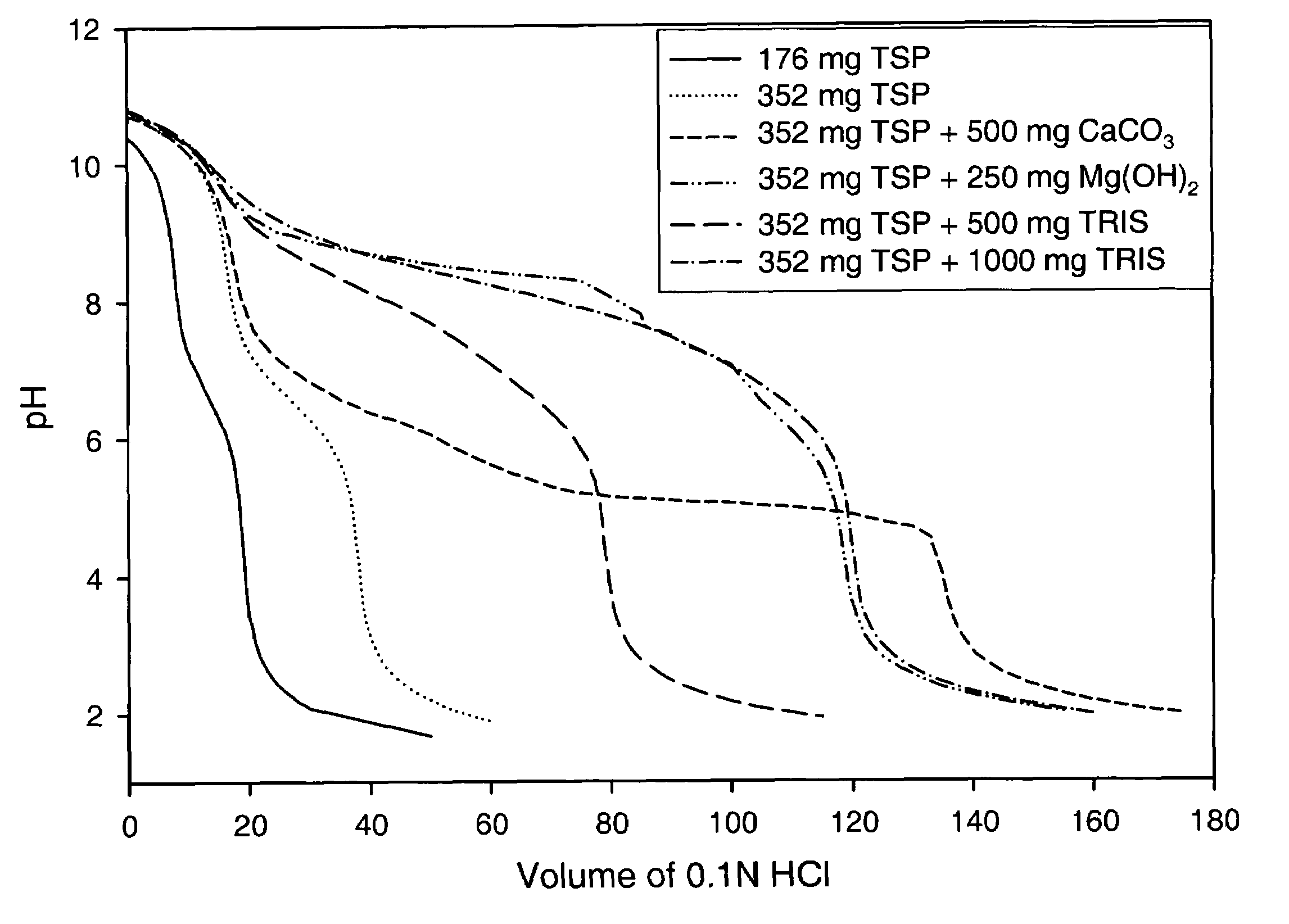
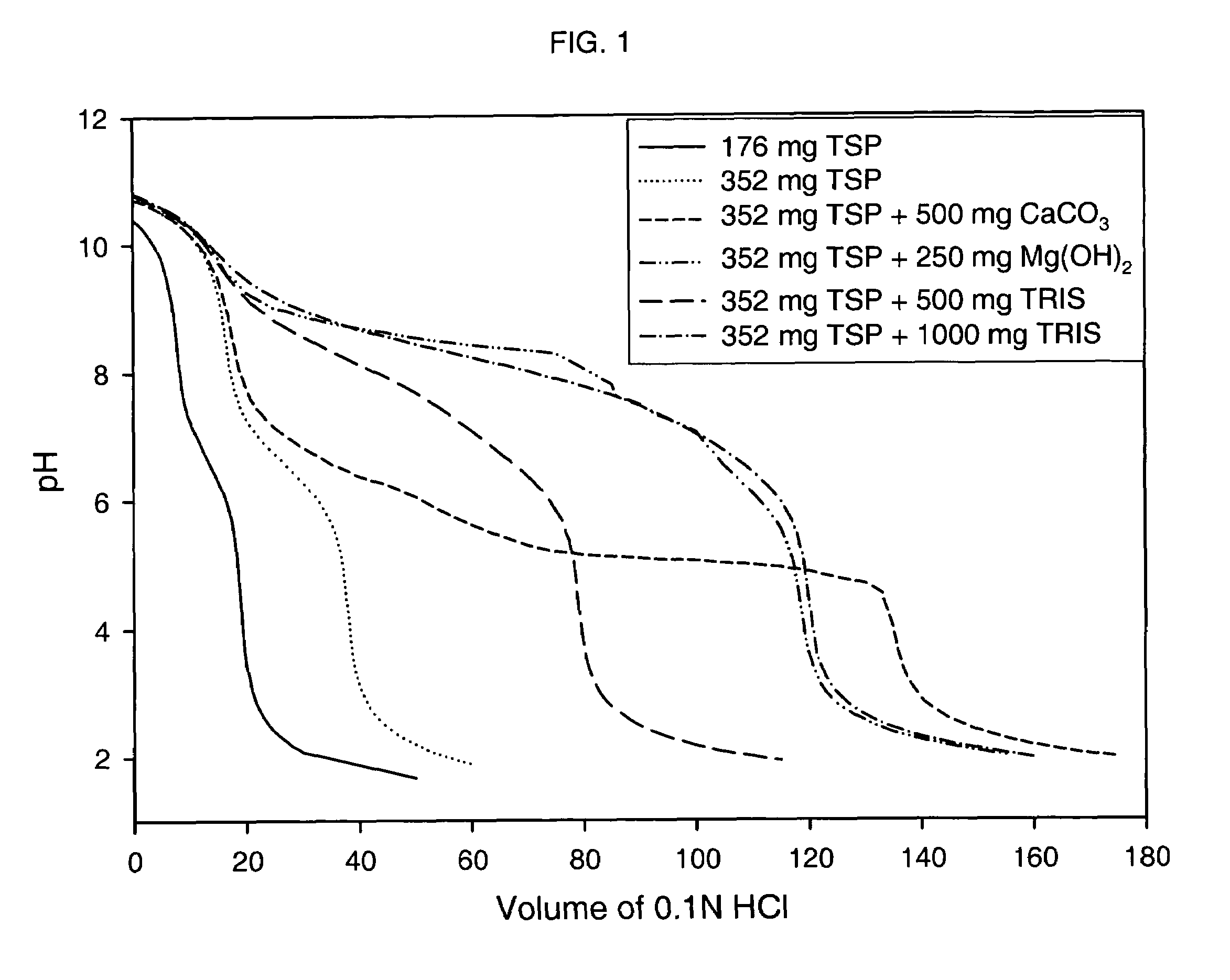
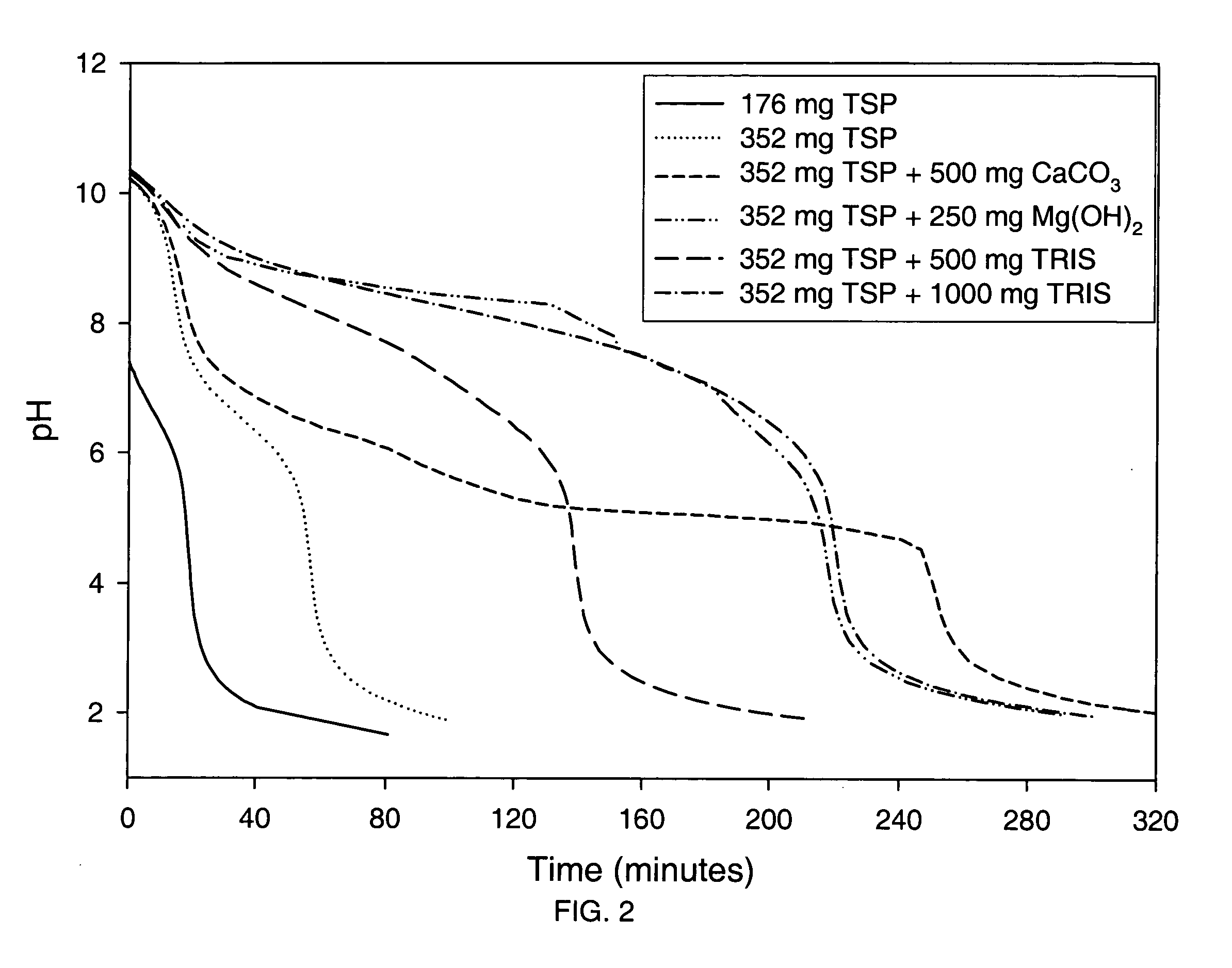
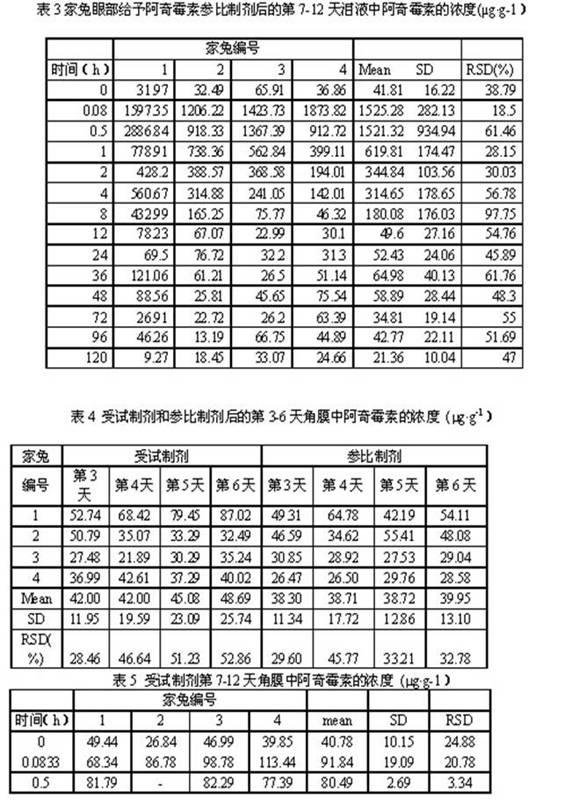
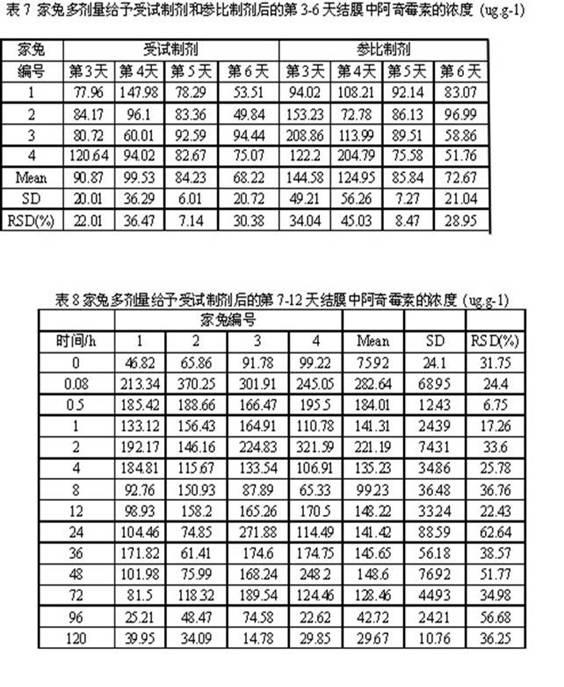
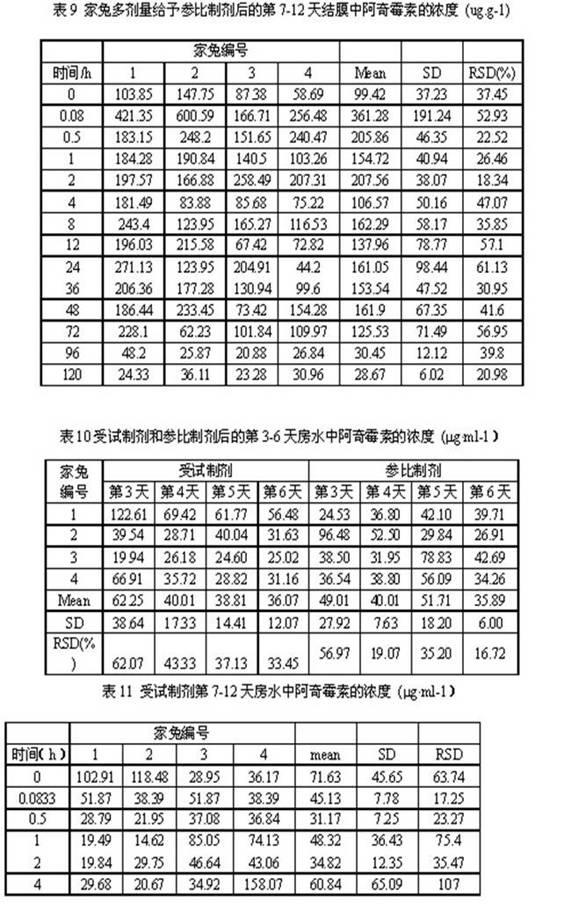
Azithromycin dosage forms with reduced side effects
ActiveUS6984403B2Reduce gastrointestinal side effectsAntibacterial agentsPowder deliveryOral suspensionsGLYCERYL MONOBEHENATE
The present invention is related to an oral dosage form comprising an effective amount of an alkalizing agent and an azithromycin multiparticulate wherein said multiparticulate comprises azithromycin, a glyceride which comprises glyceryl monobehenate, glyceryl dibehenate, glyceryl tribehenate, or a mixture thereof and a poloxamer. Typically, the oral dosage form includes any suitable oral dosing means such as a powder for oral suspension, a unit dose packet or sachet, a tablet or a capsule.
Owner:PFIZER INC
Enteric Coated Azithromycin Multiparticulates
InactiveUS20080199527A1Reduce incidenceReduce severityAntibacterial agentsPowder deliveryPharmaceutical SubstancesEnteric coating
A pharmaceutical composition is disclosed which comprises multiparticulates wherein said multiparticulates further comprise an azithromycin core and an enteric coating disposed upon said azithromycin core.
Owner:PFIZER INC
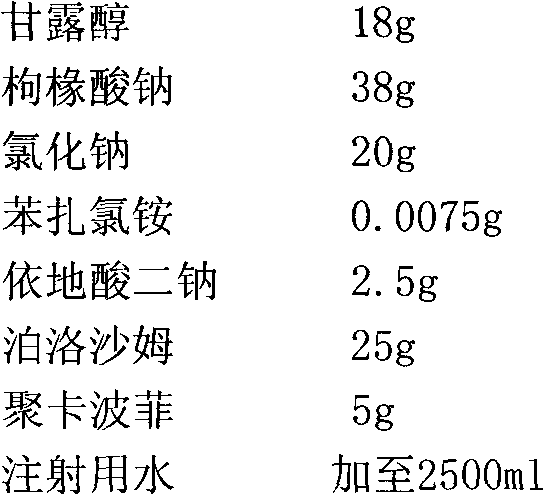
A kind of azithromycin gel eye drop and its preparation process
ActiveCN102283799AStrong practical significanceStrong application valueAntibacterial agentsOrganic active ingredientsBacterial ConjunctivitisAntioxidant
The invention discloses azithromycin gel eye drops and a preparation process thereof. The eye drops are prepared from a main medicine, namely azithromycin and excipients such as an adhesive, a gel matrix, an isotonic regulator, a preservative, an antioxidant, a buffering agent and the like. The adhesive, namely polycarbophil in the eye drops can increase the biological adhesion of the eye drops, so that the detention time of medicines in eyes is further prolonged. The invention provides a practical, convenient and reliable ophthalmic preparation for treating bacterial conjunctivitis, and solves the problems that the detention time of medicines in an eye drop formulation in the eyes is short, the medicines are not easy to absorb, the bioavailability is low and the like.
Owner:北京乐维生物技术有限公司
Method of treating dry eye disease with azithromycin
The present invention relates to a method for treating dry eye disease. The method comprises identifying a subject suffering from dry eye disease, and administering to the subject an amount of azithromycin effective to reduce dry eye symptoms and / or signs ands to improve tear film quality. Azithromycin is preferably administered topically to the subject in an aqueous ophthalmic solution comprises 0.5-1.5% (w / v) azithromycin in a polymeric suspension.
Owner:INSPIRE PHARMA
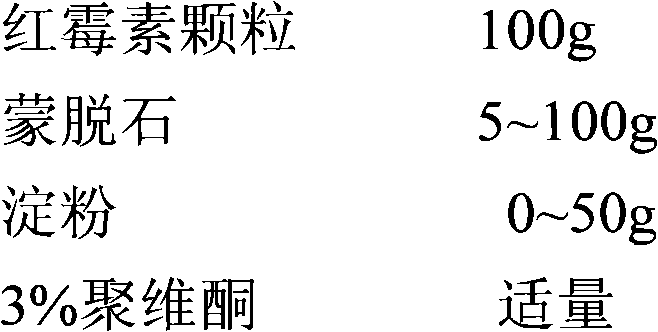
New preparation of erythrocin and relevant drug thereof and preparation method of new preparation
The invention relates to a preparation method of new preparation of erythrocin, which is characterized in that an endothelin core of erythrocin is prepared, and then an isolating layer, a protective layer, a second isolating layer and an improved enteric-coating material layer are applied one by one. In this way, new preparation of the erythrocin which has certain feature of releasing (dissolving) in acid solution (hydrochloric acid solution 9 to 1000) can be formed. The technology of the new preparation can also be widely applied to drugs which, like erythrocin, when being taken orally by a patient, cause the patient to suffer the side effects of stimulation, sickness and the like after degradation in the stomach of the patient or contact with the stomach of the patient, and drugs which the patient needs to take orally to let the blood concentration to reach the peak value in a short time. Such drugs include macrolides of azithromycin, metronidazole of nitroimidazoles, tinidazole, acyclovir as an antiviral drug, ammonium chloride as a phlegm eliminating drug, bromhexine, chloroquine as an antimalarial, nitroquine, artemisinin, dihydroartemisinin, artesunate, primaquine, pyrimethamine, carbarsone and emetine amebicides and so on.
Owner:胡昌勤 +1
Azithromycin antimicrobial derivatives with non-antibiotic pharmaceutical effect
InactiveUS20160031925A1Speed up the processReduce unnecessary use of antibioticBiocideSugar derivativesImmunomodulationsMicrobiology
The invention provides molecules, which are based on a modification of azithromycin, removing the antibiotic effect, while retaining other beneficial effects, such as, but not limited to immunomodulatory effects. The compounds of the invention can be described by compounds of Formula (I) as further defined herein.
Owner:PROBIOTIC PHARMA APS
Stabilizer of lyophilized powder injection for azithromycin injection
InactiveCN102552918AImprove stabilityReduce contentAntibacterial agentsOrganic active ingredientsAzithromycin InjectionDrug product
The invention belongs to the field of chemical drugs, and specifically relates to a stabilizer of a lyophilized powder injection for azithromycin injection. The stabilizer is characterized in that the stabilizer is prepared by mixing citric acid and sodium hydroxide, and a mass ratio of the main medicine to the stabilizer is 41-73%:26-59%. With the stabilizer of the present invention, the impurity content in the lyophilized powder injection for azithromycin injection can be effectively reduced, the drug stability can be improved, and the drug use safety can be ensured.
Owner:SHANDONG QIDU PHARMA
Azithromycin detection molecular imprinting monolithic micro column and preparation method thereof
InactiveCN104829774AImprove solubilityImprove liquid permeabilityComponent separationFunctional monomerMicro column
The invention discloses an azithromycin detection molecular imprinting monolithic micro column and a preparation method thereof, and belongs to the technical field of material chemistry. The preparation method of the azithromycin detection molecular imprinting monolithic micro column comprises the following steps: a template, a mixed pore forming agent, a functional monomer, a crosslinking agent and an initiator are polymerized at 50-80 DEG C for 48h, and the template is removed to obtain the azithromycin detection molecular imprinting monolithic micro column. The azithromycin recovery rate of the prepared molecular imprinting monolithic micro column is more than 90%, the prepared molecular imprinting monolithic micro column shows high cross reactivity, high selectivity and specificity, and has a broad application prospect in use as a purification and pretreatment material for analysis of a sample of azithromycin in animal tissues, feeds and environmental water, and other matrix.
Owner:SOUTH CHINA AGRI UNIV
Azithromycin enteric casing microsphere and its preparing process
InactiveCN1839871ASimple bittersMask bitternessAntibacterial agentsOrganic active ingredientsAntibacterial actionPlasticizer
The invention relates to enteric-coated Azithromycin microballoons and the preparing process, wherein the preparation comprises (by weight percent) Azithromycin 8-32%, macromolecular enteric-coating material 28-60%, antisticking agent 15-34% and plasticizer 5-25%.
Owner:NANJING UNIV OF TECH
Azithromycin ultrafine powder in-situ gel eye drops and preparation method thereof
InactiveCN102106812AHelps maintain effective concentrationImprove topical bioavailabilityAntibacterial agentsOrganic active ingredientsBacterial keratitisEye drop
The invention provides azithromycin ultrafine powder in-situ gel eye drops and a preparation method thereof. The method comprises the following steps of: firstly, micronizing a medicament by using a proper method to remarkably improve the dissolution rate and solubility of the medicament; and secondly, preparing an ophthalmic preparation which is in a liquid state in vitro and is in a gel state when dripped into eyes by adopting sodium alginate as an ion-sensitive gel matrix or adopting poloxamer 407 and poloxamer 188 as thermosensitive gel matrixes. The eye drops are steady in storage at 4 DEG C and uniform in content, and are not required for secondary preparation so as to reduce contamination. In the method, azithromycin ultrafine powder and in-situ gel are combined for the treatment of bacterial keratitis and trachoma; and by the dual effects of the ultrafine powder and the gel, the sustained release effect is achieved when the retention time of the preparation in the eyes is prolonged, so that the local bioavailability of the medicament and the compliance of patients are improved, and the medicament absorption of the cornea is increased to reduce the toxic or side effect caused by the systemic absorption of ophthalmic medicaments.
Owner:SHENYANG PHARMA UNIVERSITY
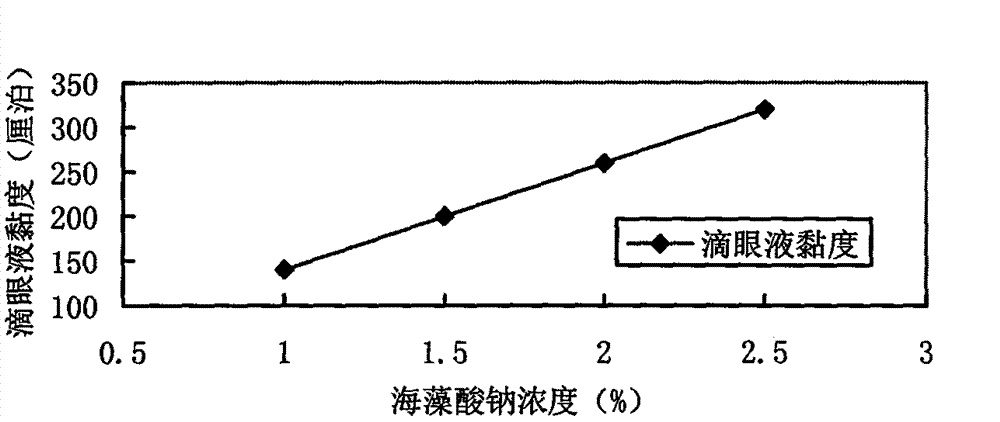
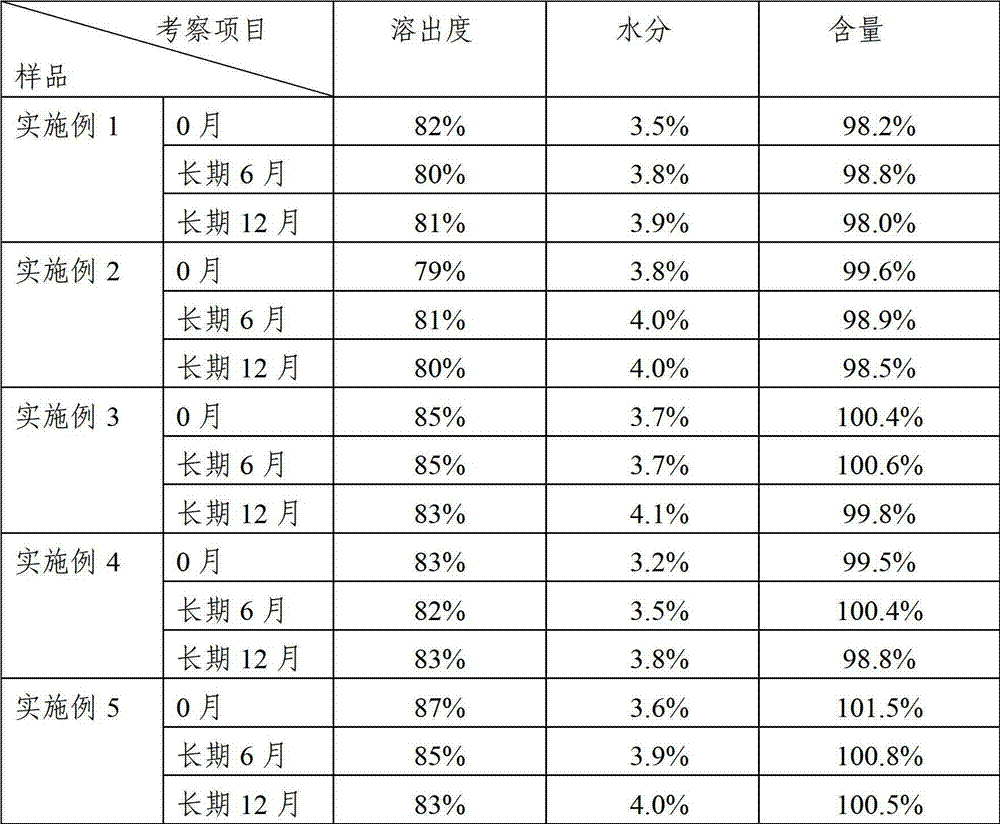
Azithromycin eye drops and preparation method thereof
InactiveCN102824305AAntibacterial agentsOrganic active ingredientsDrugs preparationsPharmaceutical drug
The invention relates to the field of medicinal preparations and specifically to Azithromycin eye drops which are characterized by containing Azithromycin, a matrix, a stabilizing agent, a pH value conditioning agent, an antiseptic and injection water, wherein the matrix is sodium alginate. The invention also discloses a preparation method for the eye drops. According to the invention, sodium alginate is used as the hydrophilic matrix, so good biocompatibility is obtained; the eye drops are in a form of a semi-colloidal preparation under the condition of in vitro storage; and the preparation has appropriate viscosity and does not run off when dropped into eyes, so the preparation can substantially prolong action time of drugs in the eyes, reduce administration frequency and improve compliance of patients.
Owner:南京恒道医药科技股份有限公司
Azithromycin derivative and its use
InactiveCN101074250ASugar derivativesNitro compound active ingredientsAzithromycinBACTERIAL INFECTIOUS DISEASES
An azithromycin derivative, its medicinal acceptable salt and its usage are disclosed. In structural formula of medicinal acceptable salt, IR is -R1 or -A-R1; A is C1-5 alkylene, C2-5 vinylene, C2-5 acetylene, C3-6 naphthelene, 3-6 valence heterocyclene or C6-10 arylene containing 1-2 selected from N, O and S hetero-atom; R1 is 5-15 valence aromatic nucleus containing 0-3 selected from N, O and S hetero-atom, which can be substituted by various substituting group. It can be used to prepare medicines in treatment and prevention of infectious diseases.
Owner:SHANGHAI INST OF PHARMA IND
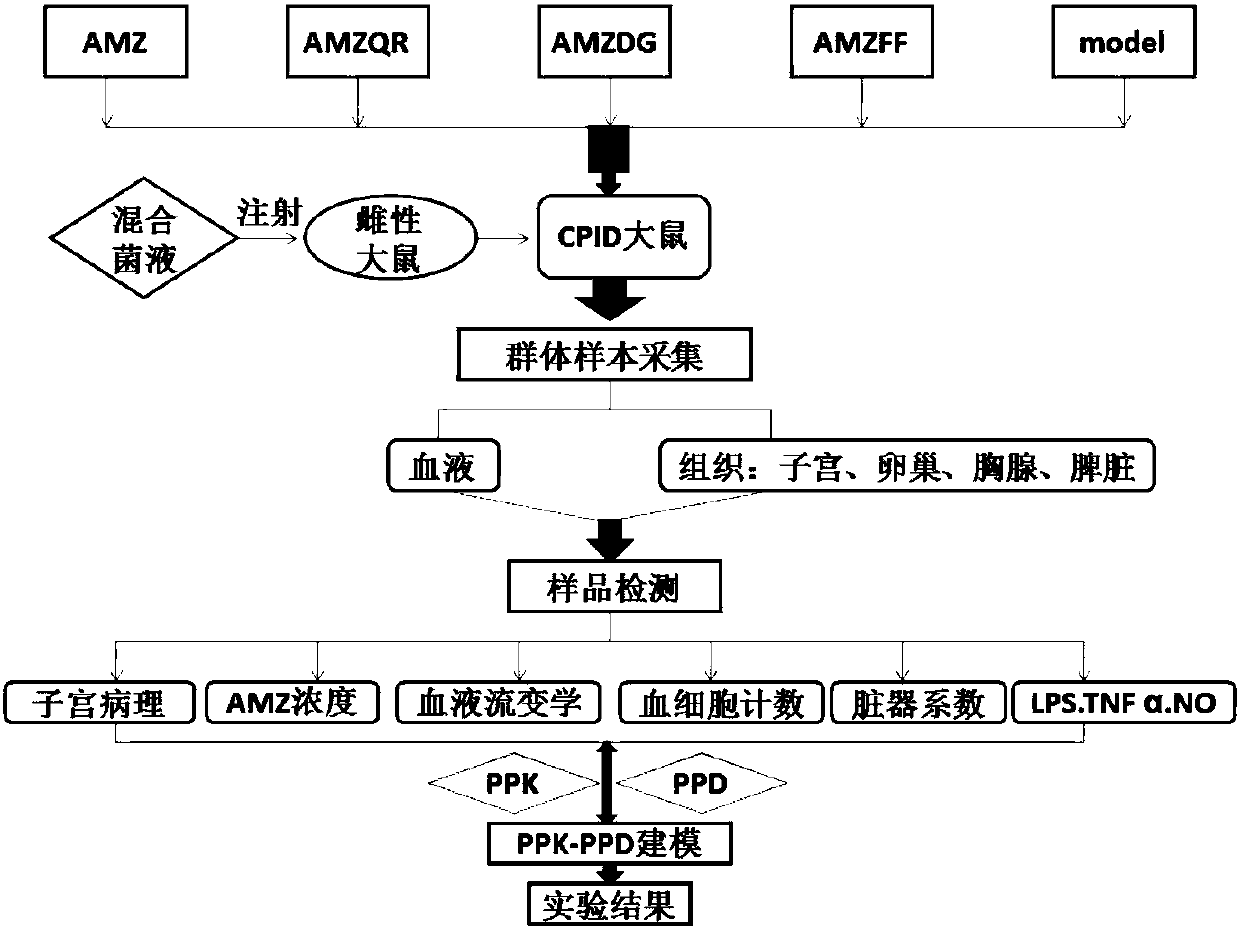
Evaluation method for improvement effect of traditional Chinese medicine component on azithromycin action baseline, and applications of evaluation method in evaluation of Fukeqianjin prescription
ActiveCN107064480AImprove product qualityQuantitative contribution rateComponent separationBiological testingTherapeutic effectAntibiotic Y
The invention discloses an evaluation method for the improvement effect of a traditional Chinese medicine component on an azithromycin action baseline, and applications of the evaluation method in evaluation of a Fukeqianjin prescription. According to the present invention, a baseline equal addition method is used, wherein the action level of the exact and effective treatment drug azithromycin is determined, the azithromycin and a tested traditional Chinese medicine composition are administered to experiment models in a parallel manner, the baseline level of the combination medication and the treatment effect trend of the of the combination medication are determined, a group pharmacokinetics-group pharmacodynamics combined model is established, and verification is performed; the pharmacokinetics-pharmacodynamics system variation dynamics system capable of quantitatively evaluating the participation of the medicinal guide is scientifically designed, and the disadvantage of the application of the weak efficacy drug treatment effect index point method to determine mean value comparison in the research method is changed; with the application of the evaluation method of the present invention in the evaluation of the improvement of the Fukeqianjin prescription, the synergetic effect of the Fukeqianjin prescription composition on each pharmacokinetic parameter of the azithromycin can be defined, and the main drug group providing effects of anti-inflammation and blood circulation activating in the Fukeqianjin prescription composition can be summarized and obtained; and the evaluation method can be well used for evaluating the safety and the effectiveness of the combination of the traditional Chinese medicine composition and the antibiotics, particularly for evaluating the internal elimination effect of the angelica sinensis component medicinal guide in the Fukeqianjin prescription.
Owner:ZHUZHOU QIANJIN PHARMA
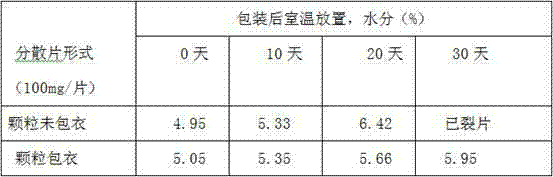
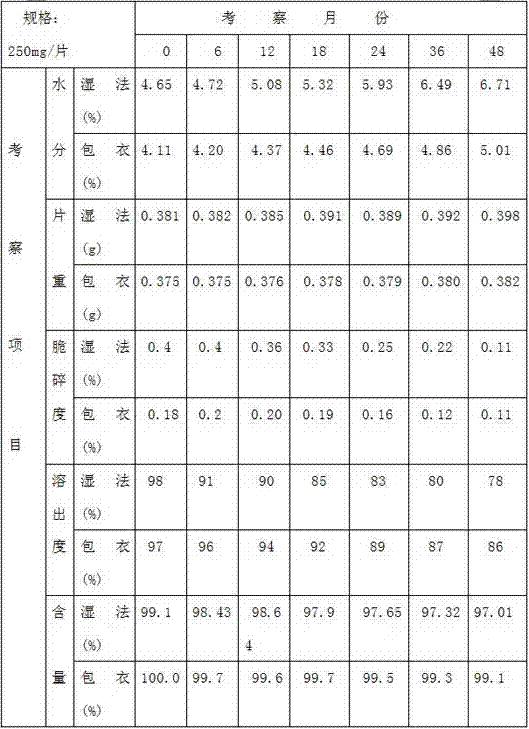
Preparation method of azithromycin dispersible tablets
InactiveCN105193753AHigh dissolution rateImprove cleanlinessAntibacterial agentsOrganic active ingredientsExperimental researchEngineering
The invention discloses a preparation method of azithromycin dispersible tablets. The preparation method comprises the following steps: weighing, preparation of an adhesive, preparation of wet granules, drying and total blending. The process obtained through large amount of experimental research on the prescription of the dispersible tablets solves the problems of sticking, tablet cracking, uncapping and the like of azithromycin in a tablet compressing process, and the dispersible tablets with uniform dispersion, good dissolution, smoothness, uniformity and good cleanliness are obtained. The method is simple to operate, has low requirement for equipment, facilitates preparation and is suitable for industrial mass production.
Owner:CHENGDU TONGDE PHARMA
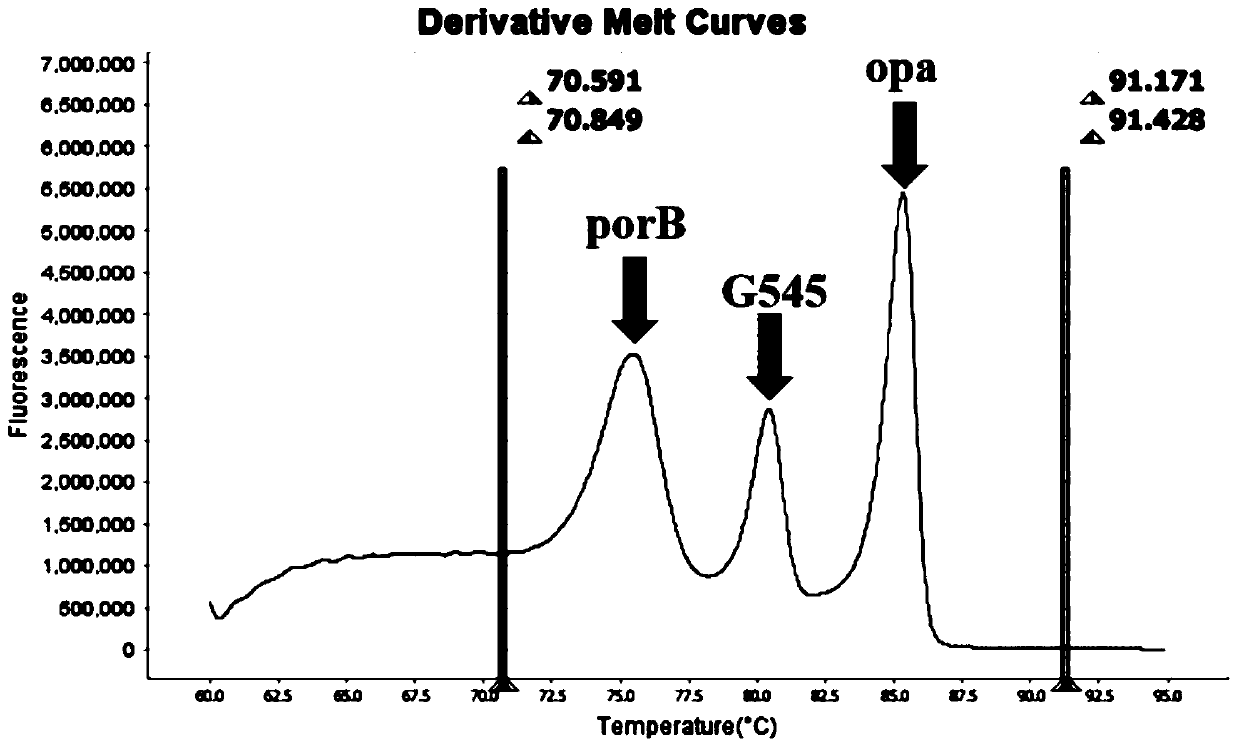
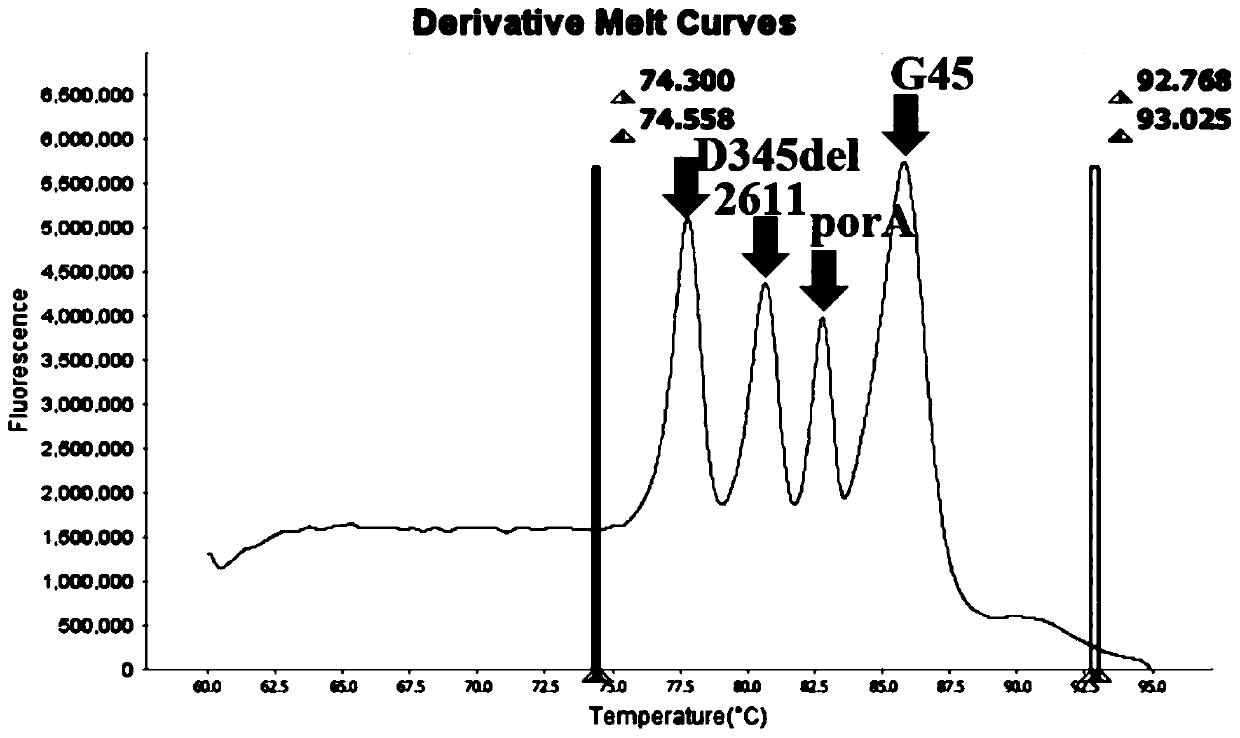
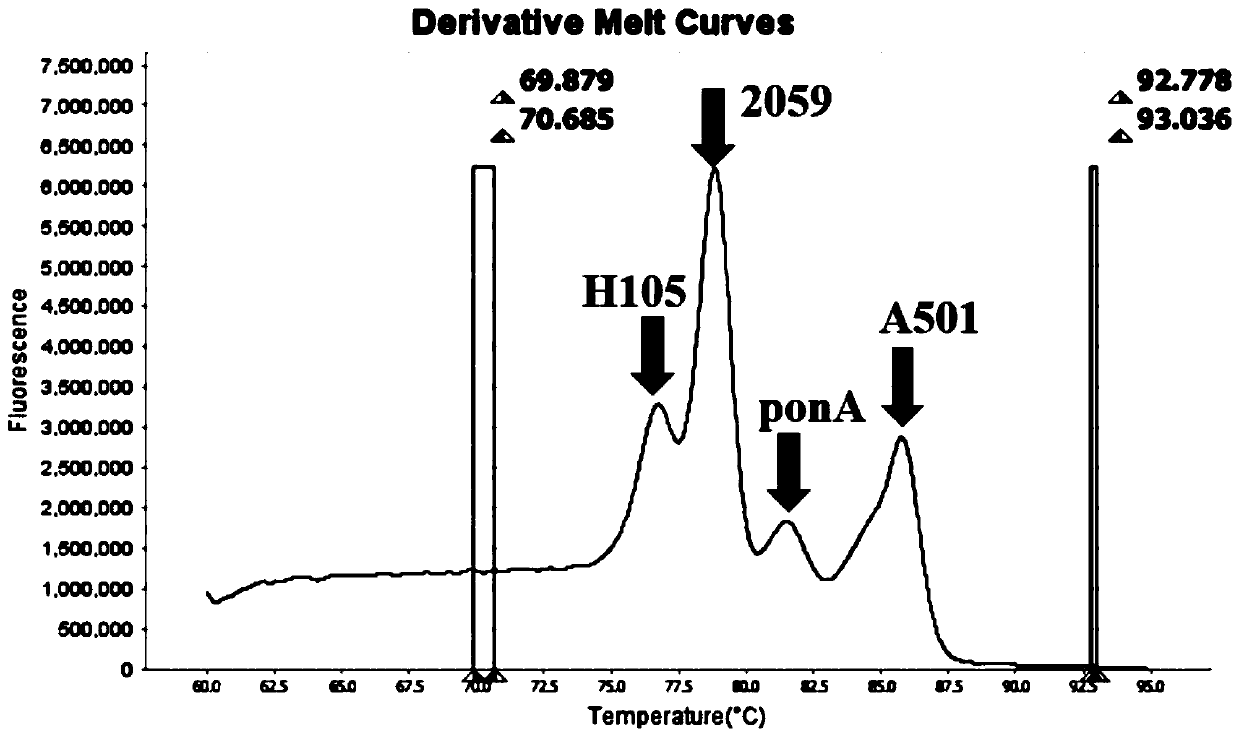
Method and kit for multiple detection of drug resistance sites of neisseria gonorrhoeae
PendingCN110643722AFast and Sensitive AnalysisImprove accuracyMicrobiological testing/measurementMicroorganism based processesHigh Resolution Melt AnalysisRelated gene
The invention belongs to the technical field of molecular biology detection, and relates to a detection method for drug resistance sites, in particular to a method and a kit for multiple detection ofthe drug resistance sites of neisseria gonorrhoeae. A provided specific primer for the multiple detection of the drug resistance sites of the neisseria gonorrhoeae selects drug-resistance-related genes of two drug combinations (ceftriaxone and azithromycin) as target genes for detection, and includes penA, ponA, porB, mtrR, and 23S rRNA. Based on high resolution melting curve analysis technique, the DNA demulsification process is monitored in real time through high resolution melting of PCR products, the mutation condition of the genes is analyzed according to the characteristic change of a melting curve, and thus a basis is provided for determining the drug-resistant condition of the neisseria gonorrhoeae.
Owner:USTAR BIOTECHNOLOGIES (HANGZHOU) CO LTD
Azithromycin capsule and preparation method thereof
InactiveCN103040787AEvenly dispersedOvercome water absorptionAntibacterial agentsOrganic active ingredientsAzithromycinMedicine
The invention relates to an azithromycin capsule which comprises azithromycin capsule comprises azithromycin, a filling agent, an adhesive and a glidant, and further relates to a preparation method of the azithromycin capsule. In the technical scheme, raw materials are made into a micropill capsule, and medicines are uniformly dispersed, so that the problem that the main medicine has low probability of absorbing water is solved, the product quality is improved, and the medicine stability is guaranteed. The capsule has the advantages of high dissolution speed and high bioavailability, and the preparation method provided by the invention is simple to operate, is under mild conditions and is suitable for large-scale industrial production.
Owner:蚌埠丰原涂山制药有限公司
Production method of sterile stable azithromycin eye drops
InactiveCN103211755AImprove stabilityImprove securityAntibacterial agentsPowder deliveryAzithromycinFreeze-drying
The invention discloses a production method of sterile stable azithromycin eye drops. The sterile stable azithromycin eye drops comprise a sterile solid drug packaged individually and a special-purpose solvent for dissolving the sterile solid drug. The sterile solid drug contains an active component and pharmaceutically acceptable auxiliary materials. The active component is azithromycin. According to the production method, the active component azithromycin and the pharmaceutically acceptable auxiliary materials are prepared into sterile freeze-dried powder and the sterile freeze-dried powder is dissolved in the special-purpose sterile solvent in clinical use. The production method can improve stability of the azithromycin eye drops, realize production of the sterile stable azithromycin eye drops, and improve safety of the sterile stable azithromycin eye drops in use.
Owner:WUHAN NUOAN PHARMACY
Preparation method of animal antibiotic tulathromycin
ActiveCN103588833AHigh purityAvoid Pd/CSugar derivativesSugar derivatives preparationBiotechnologyAcetic anhydride
The invention belongs to the technical field of organic synthesis and pharmaceutical chemistry and especially relates to a preparation method of an animal antibiotic tulathromycin. According to the preparation method, azithromycin A and acetic anhydride which are used as raw materials are subjected to protection and oxidation to obtain oxide cyclic ketone; the intermediate is used for innovative addition of cyclic ketone and nitromethane; an addition product is reduced to obtain methyleneamine; direct condensation between methyleneamine and propionaldehyde is carried out; and reduction is conducted to obtain the high-purity target compound. Raw materials which are cheap and easily available are adopted. The preparation method has advantages of simple reaction, easily-controlled reaction process, high product purity, manageability, high yield, low cost and the like, and is suitable for large-scale industrial production.
Owner:UNIV OF SCI & TECH BEIJING
Preparation method of azithromycin fine granule
InactiveCN101416945ASweet tasteEasy to acceptAntibacterial agentsOrganic active ingredientsAzithromycinFood additive
The invention pertains to a preparation method adopting a spray-drying method to encapsulate drugs into miniature capsule fine granules, more particularly relates to the preparation of azithromycin fine granules. The preparation method comprises the steps that: (1) poly-acrylic acid resin is soaked in an organic solvent, stirred, dissolved and prepared into a poly-acrylic acid resin solution after being filtered by a mesh sieve; (2) azithromycin material is added into the poly-acrylic acid resin solution obtained from Step 1), thus obtaining a mixed material; (3) the mixed material of Step (2) is treated with spray-drying, and miniature capsules are collected; and (4) the miniature capsules obtained from the Step (3) are added with a sweet food additive, mixed evenly, dried and prepared into granules. The preparation method prepares the azithromycin material solution into the miniature capsules by the way of spray-drying and solves the disagreeable taste effect caused by the bitterness of azithromycin preparations by sweet layers wrapping the outer layers of the miniature capsules. The solving of the taste of the azithromycin preparations cause the granules to be convenient for users to take, especially for children and patients with chronic diseases and needing to take the granules for a long term.
Owner:沈阳金龙药业有限公司
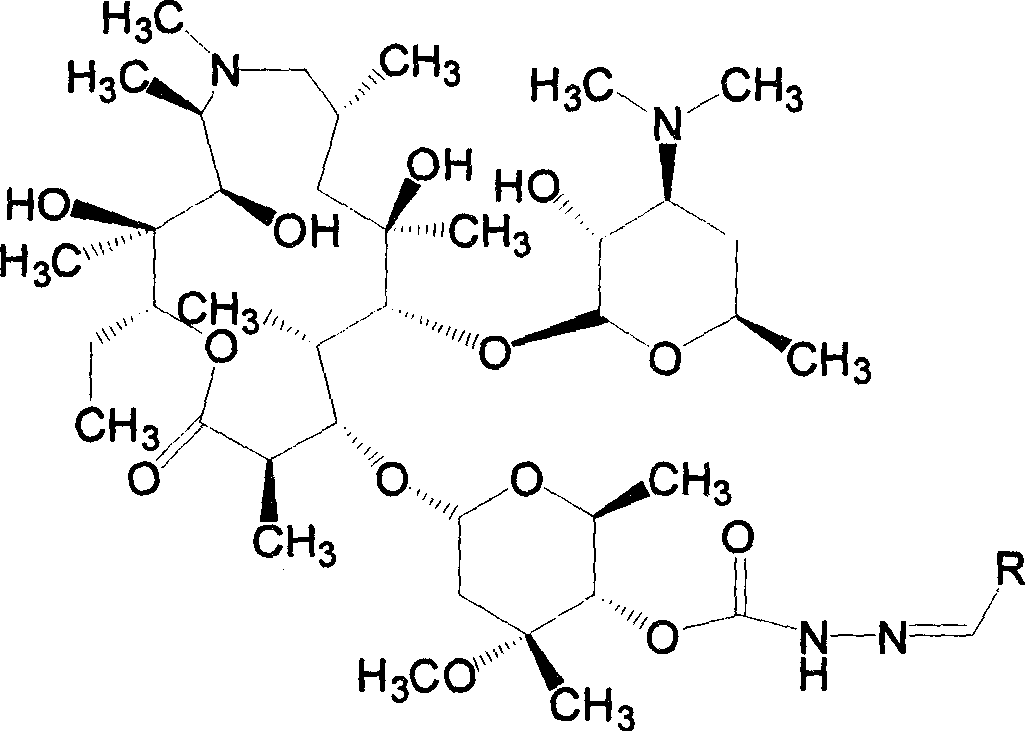
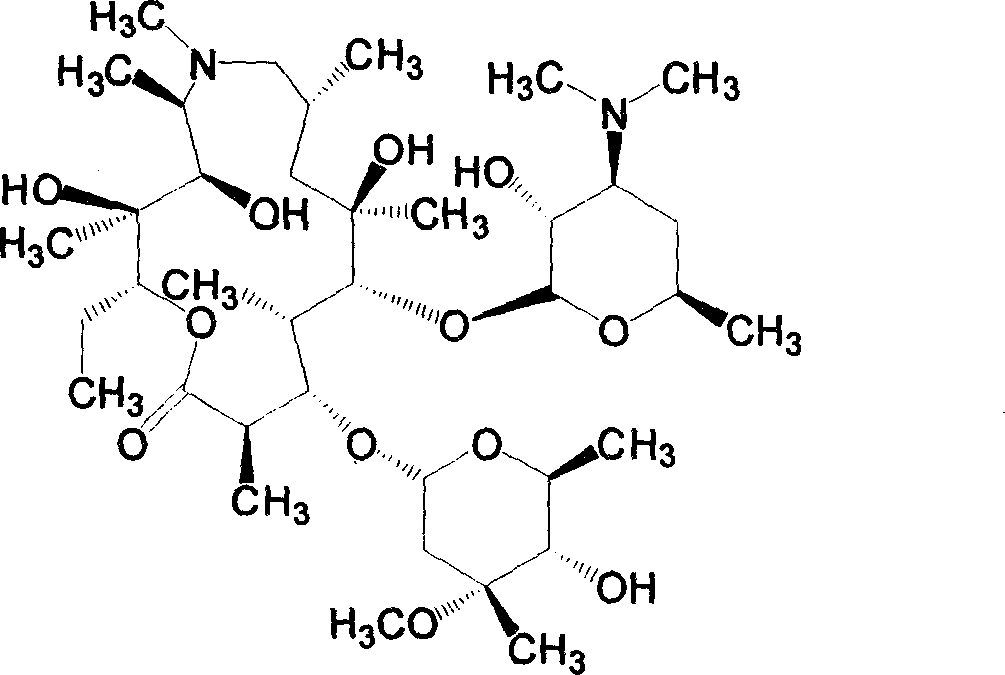
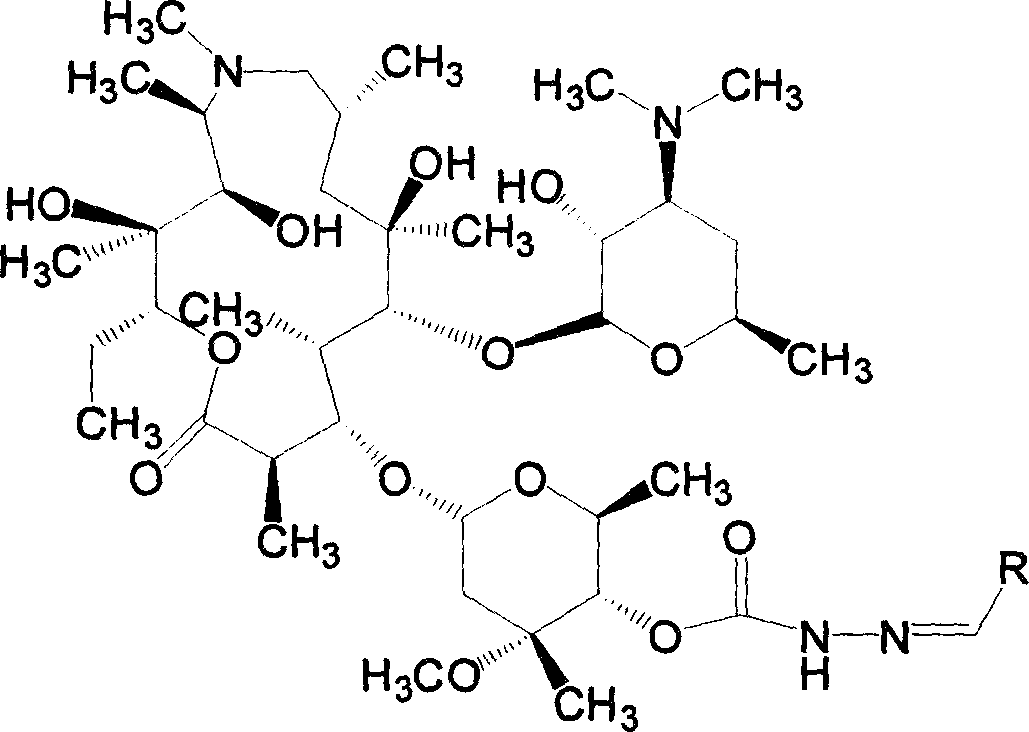
Azithromycin derivative, preparation method and intermediate thereof
InactiveCN101899076AStrong inhibitory activityAntibacterial agentsOrganic active ingredientsAzithromycinOrganic acid
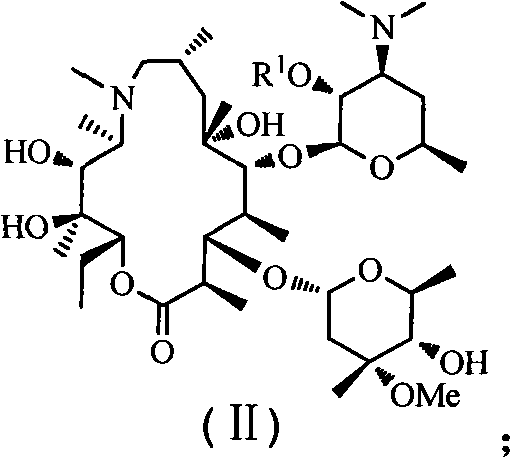
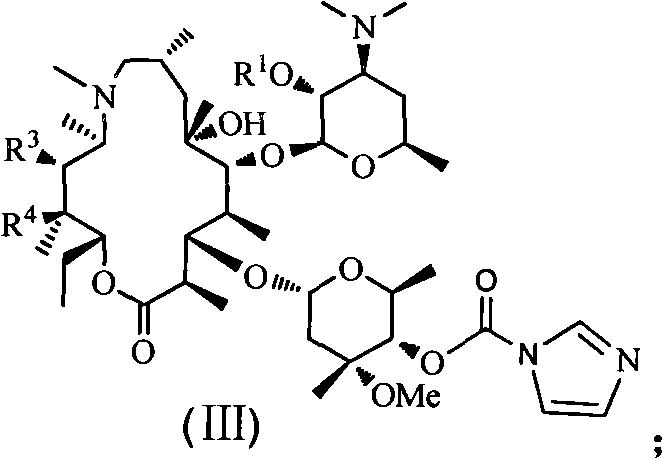
The invention discloses an azithromycin derivative shown in the formula (I) and a pharmaceutically acceptable salt thereof, wherein R1 represents hydrogen, acetyl or benzoyl, R2 represents hydrogen, aliphatic hydrocarbon, substituted aromatic alkyl, substituted aromatic heterocyclic alkyl, halogen, nitryl, alkoxy or-AR7, R3 represents OR5, R4 represents hydroxyl; or, R3 and R4 can form a ring with a structure that X represents oxygen and nitrogen; R5 represents nitrogen or CONHR6, R6 represents aliphatic hydrocarbon, substituted aromatic alkyl or substituted aromatic heterocyclic alkyl, and R7 represents aliphatic hydrocarbon, substituted aromatic alkyl or substituted aromatic heterocyclic alkyl; n is from 1 to 6, A is one of -O-,-CO-,-CONR8-,-NHCO- and -S-. The invention further relates to a prepared intermediate and a method thereof, an acceptable salt thereof forming with inorganic or organic acid, a drug composite and application thereof in the treatment of bacterial infection.
Owner:SHANDONG UNIV
Preparation method of azithromycin for injection
InactiveCN111544399AEasy to prepareReduce scrap rateAntibacterial agentsPowder deliveryDry powderAzythromycin
The invention discloses a preparation method of azithromycin for injection and belongs to the technical field of medicines. By mixing and dissolving water for injection, a cosolvent and azithromycin,and performing pre-freezing, five-stage sublimation drying and desorption drying, azithromycin freeze-dried powder for injection is obtained. In the sublimation drying process, the temperature is in arising trend, and the vacuum degree is in a rising-falling trend. The preparation method is simple, the original equipment does not need to be replaced or improved; in the preparation process, the pre-freezing temperature is reduced, five-stage sublimation drying is adopted, and the specific temperature and vacuum degree of sublimation drying are improved, so that rejection rate is reduced, yieldis increased, and production costs are further effectively saved in comparison with the traditional process; and the obtained product is easily dissolved in water for injection and has good stabilityand redissolution rate.
Owner:福州华为医药技术开发有限公司
Injectable antibiotic formulations and use thereof
ActiveUS10286003B2Organic active ingredientsPharmaceutical delivery mechanismAzithromycinMacrolide resistance
Provided herein are pharmaceutically acceptable compositions containing macrolide antibiotics, in particular azithromycin. In particular, compositions containing azithromycin with low toxicity, especially for administration to felines, are provided herein.
Owner:DECHRA VETERINARY PROD
Clathrate of azithromycin hydrate with 1,2-propyleneglycol, method for the manufacture thereof and pharmaceutical composition comprising same
Owner:HANMI PHARMA
Methods of stabilizing azithromycin
InactiveUS20080149521A9Improve stabilityPackaging corrosive chemicalsPill deliveryEngineeringCoprecipitation
A method of packaging of azithromycin which provides improved stability of azithromycin upon storage. Additionally, compositions and methods of stabilizing azithromycin compositions are described. Stabilized azithromycin compositions comprise an intimate admixture of azithromycin and a stabilizing-effective amount of an antioxidant to improve the resistance of the azithromycin to degradation. Coprecipitation or co-milling of azithromycin and an antioxidant are particularly preferred means of achieving an intimate admixture. Pharmaceutical formulations comprising a stabilized azithromycin composition and methods of making such formulations are also described.
Owner:TEVA PHARM USA INC
Paeoniflorin crystallization process with controllable crystal form and granularity
InactiveCN101418026BImprove adsorption capacityGood hygroscopicitySugar derivativesSalting outPhysical chemistry
Owner:NANJING TECH UNIV
Azithromycin sustained release tablets and method of preparing the same
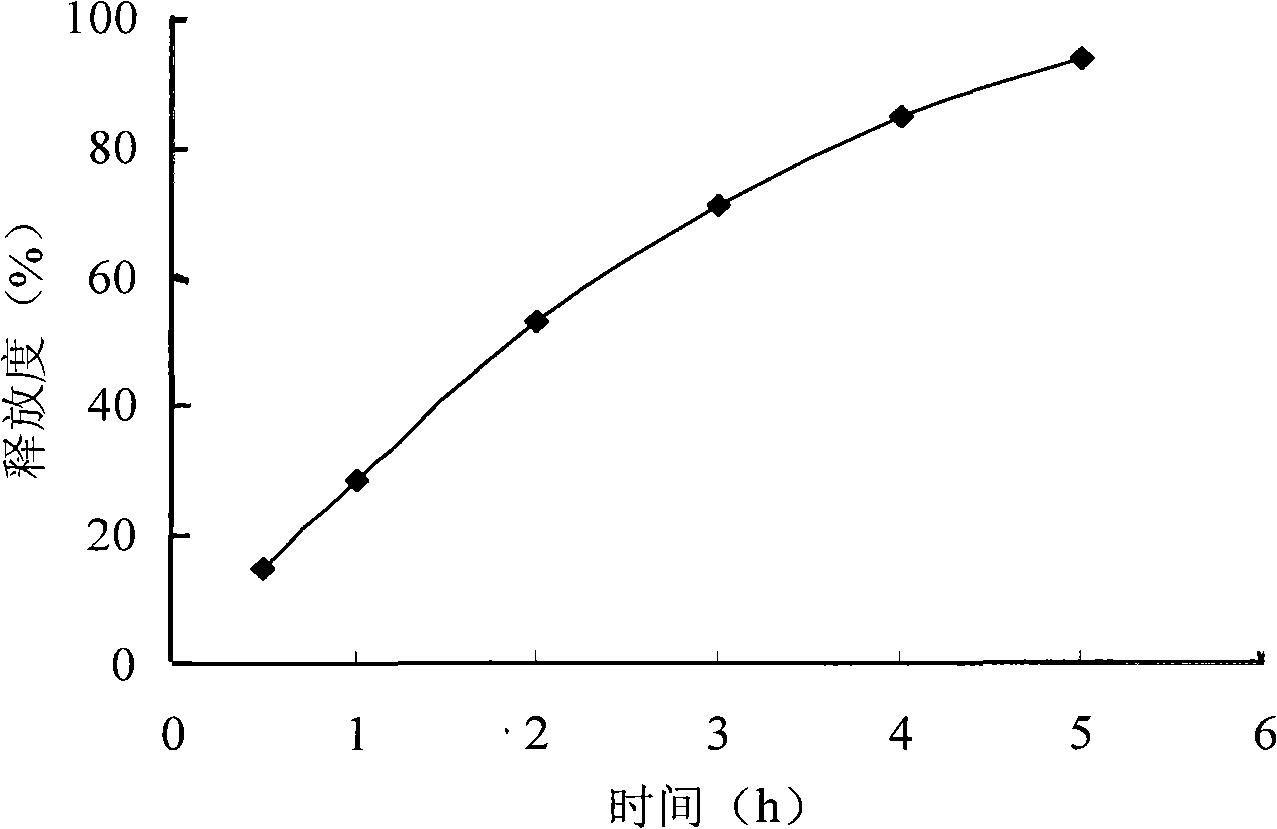
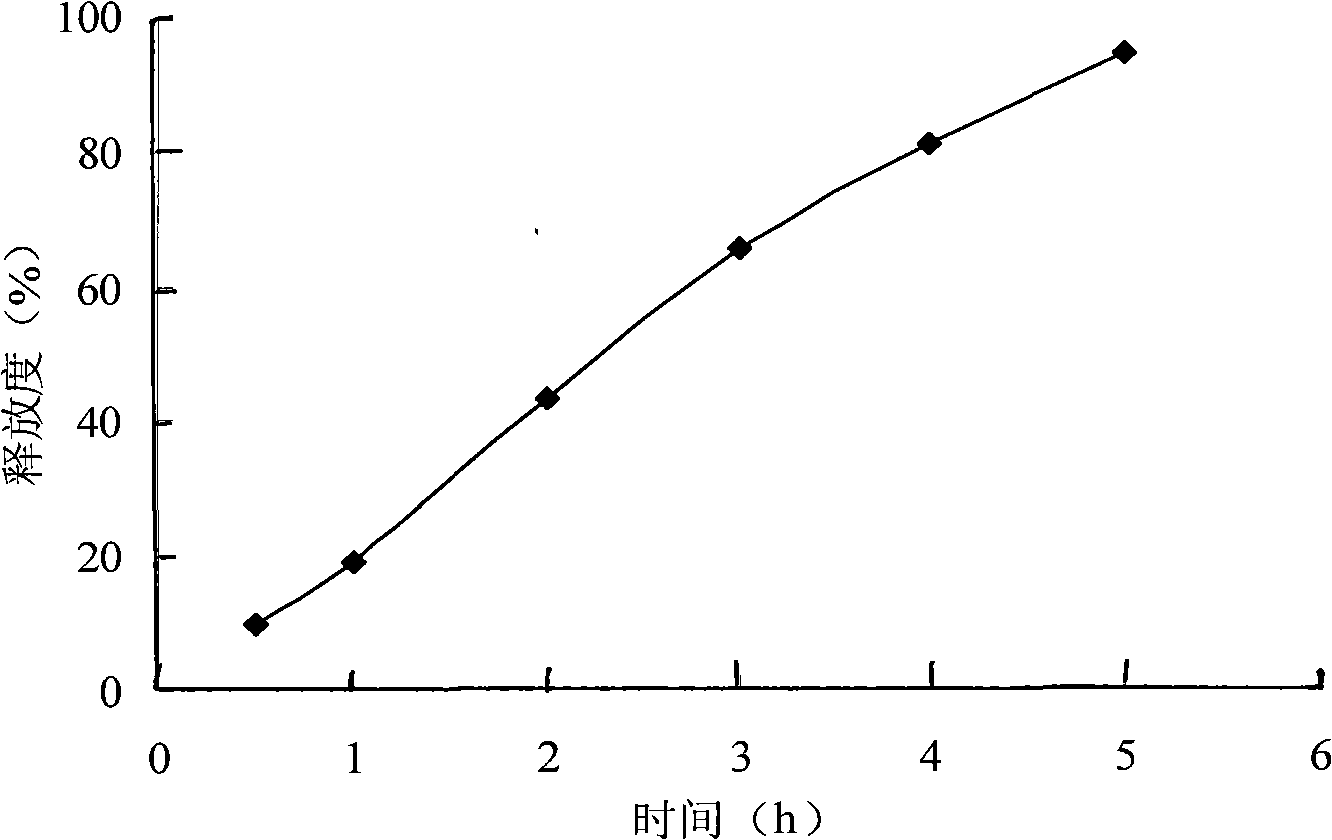
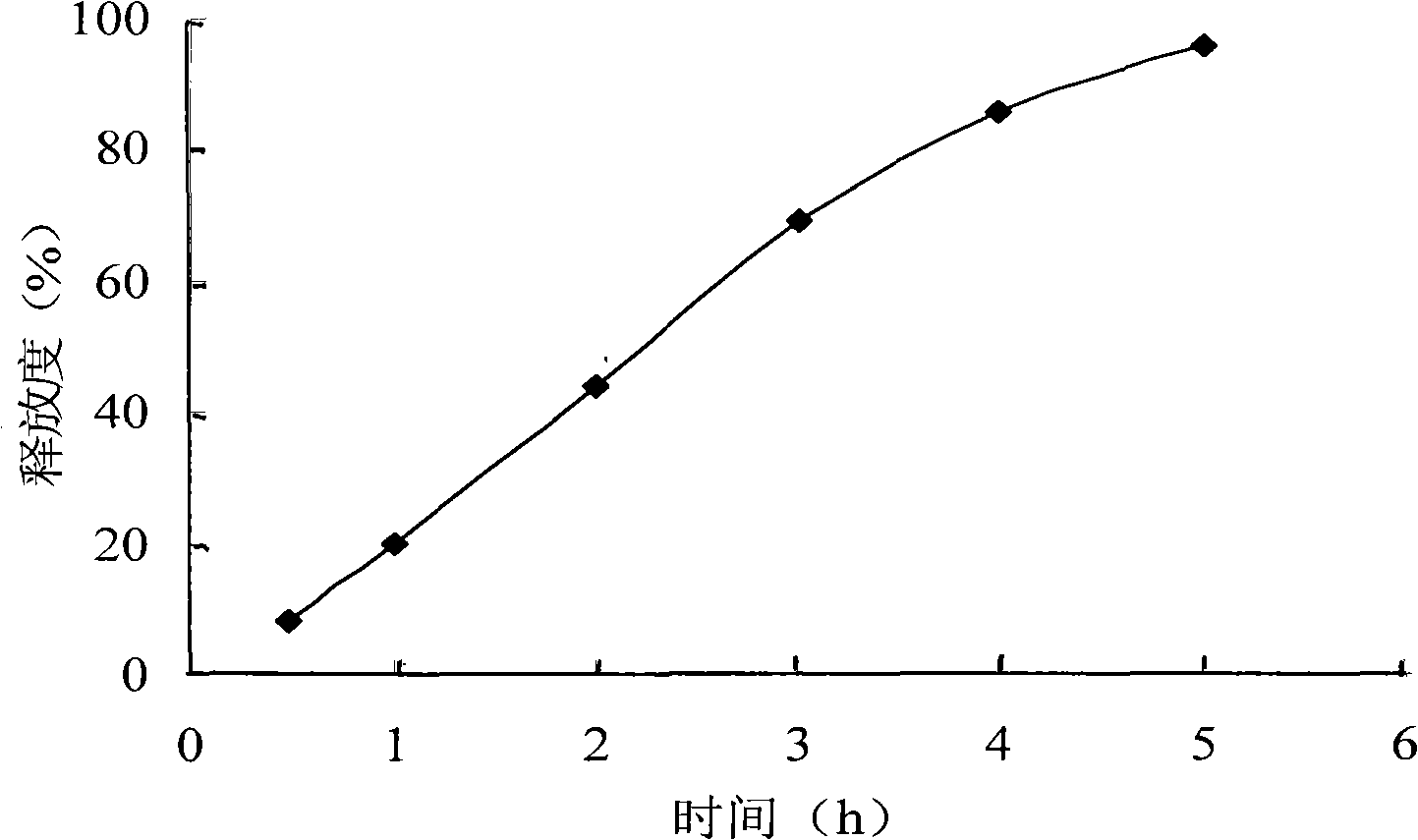
InactiveCN101278918APromote oral absorptionLittle side effectsAntibacterial agentsOrganic active ingredientsAzithromycinSide effect
The invention pertains to the technical field of medicament and discloses azithromycin sustained release tablets and a preparation method thereof. The composition of a prescription of the azithromycin sustained release tablets is as follows: 10%-80% of azithromycin, 5%-50% of sustained release formulation, 1%-50% of bulking agent and 0.1%-5% of lubricant. The invention is prepared by the following method: the azithromycin, the sustained release formulation, the bulking agent are crushed, then passes 100 mesh sieve and mixed evenly; binder is added to make damp mass which is sieved for pelletization; a wet pellet is dried at the temperature of 50-80 DEC C; the dried pellet is sieved for granulating and then mixed with lubricant for tabletting. The invention is capable of reducing the higher peak concentration of the azithromycin and decreasing fluctuation and untoward response of a medicine, improving medicine concentration in tissue, lengthening the half-life period of the medicine, improving the compliance of a patient, thereby reducing the occurrence of medicine resistance to the utmost extent. The prescription is capable of reducing the side effect of the azithromycin, improving bioavailability, which has the advantages of lasting drug effect and being convenient for being taken.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of azithromycin freeze-drying agent for injection
PendingCN111803455AImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsActivated carbonSolvent
The invention provides a preparation method of an azithromycin freeze-drying agent for injection. The method comprises the following steps of S1, mixing and dissolving raw materials: adding a cosolvent into water for injection, adding a pH regulator to regulate the pH value to 5.0-5.2, adding azithromycin into the water for injection, and performing stirring until the azithromycin is dissolved toform a mixed liquid medicine A; S2, performing decolorizing and impurity removing: regulating the pH value to 6.0-7.0 by adopting the pH regulator, adding activated carbon for needles, performing stirring for 15-20 min at the room temperature, and performing sterilizing, filtering and decarbonizing to form a mixed liquid medicine B; and S3, performing freeze-drying: a, repeatedly performing pre-freezing, b, performing primary sublimation drying, and c, performing drying again, wherein the cosolvent is citric acid, sodium hydroxide, mannitol and cis-6-nonen-1-ol, and the raw materials include the following components in parts by weight: 100 parts of the azithromycin, 50-60 parts of the citric acid, 20-30 parts of the sodium hydroxide, 30-40 parts of the mannitol, 3-5 parts of the cis-6-nonen-1-ol and 1500-2000 parts of the water for injection. The preparation method of the azithromycin freeze-drying agent for injection is good in solubility and high in stability, and the clarity of a prepared injection solution is high.
Owner:湖北潜龙药业有限公司
Preparation method of azithromycin dispersible tablet granule coating
InactiveCN103040776AGood film formingImprove plasticityAntibacterial agentsOrganic active ingredientsCellulosePolythylene glycol
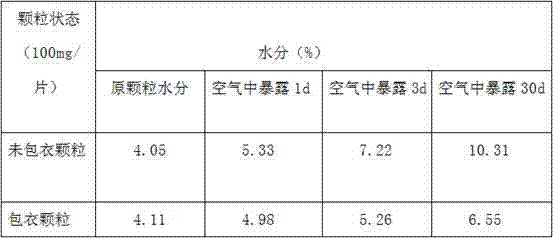
The present invention discloses a preparation method of azithromycin dispersible tablet granule coating. The granule coating process is added after mixing the materials and granulating. The coating materials are three or more selected from the group consisting of hydroxypropyl methyl cellulose, ethyl cellulose, methyl cellulose, polyvinylpyrrolidone, and polyethylene glycol. The preparation method comprises the steps of: (1) preparing the coating solution, (2) granulating, (3) coating the granules, and (4) preparing the dispersible tablets. The present invention overcomes the problems that the finished dispersible tablets are ugly in appearance caused by serious moisture absorption in the conventional preparation process of azithromycin dispersible tablets, the production cost is high, and the storage conditions are high. Thus the drug production costs are reduced. The finished products can reach moistureproof requirements by just ordinary plastic packaging. The composite membrane moistureproof protection for the finished product can be abolished.
Owner:SICHUAN MEDCO PHARML
Medicinal slow release agent for treating rabbit infections rhinitis and its preparing method
InactiveCN1943587AHigh inclusion rateImprove securityAntibacterial agentsPowder deliveryAzithromycinAlcohol
The invention discloses a slow-released preparation in treatment and prevention for rabbit malignant rhinitis and means of making thereof, belonging to the technical field for treating and prevention of livestock epidemic disease. Said preparation consists of Azithromycin and beta cyclodextrin as materials and prepares in accordance with part by weight; preparation means is, first mixing and dissolving Azithromycin and anhydrous alcohol in accordance with weight-to-volume ratio : 1g.: 7-9 ml.; mixing and grinding into paste beta cyclodextrin and distilled water in accordance with weight-to-volume ratio : 1g.: 0.5-1.5ml.; thereafter mixing, compounding, drying, grinding both described hereinabove to obtain slow-released powder; being prepared into injection when applying.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Azithromycin soft capsules and preparation method thereof
ActiveCN101664396ANo precipitationDissolve completelyAntibacterial agentsOrganic active ingredientsCitric acidPEG 400
The invention discloses azithromycin soft capsules and a preparation method thereof. The soft capsules comprise the following raw material components: azithromycin, polyethylene glycol 400, polyethylene glycol 200, water, acetic acid, 1,2-propanediol and the like. The soft capsules are prepared through the steps of stirring, pressing and the like in the preparation process. Experiments show that the azithromycin soft capsule preparations prepared by the preparation method have stable solution without precipitate, and particularly the acetic acid replaces the commonly used lactic acid, citric acid or lactobionic acid and other cosolvents, and the conventional process is changed by adding water in the process of preparing the soft capsules, and thus the content of the prepared soft capsule has no precipitate, is completely dissolved and has good stability to ensure the quality of the content of the capsule.
Owner:HAINAN HUALON PHARM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com