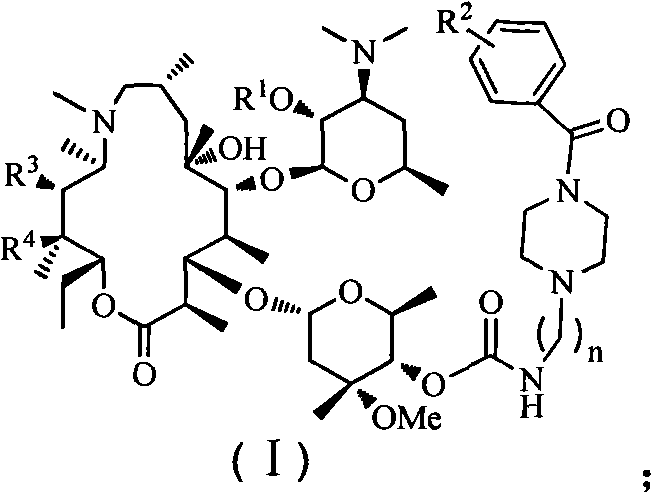
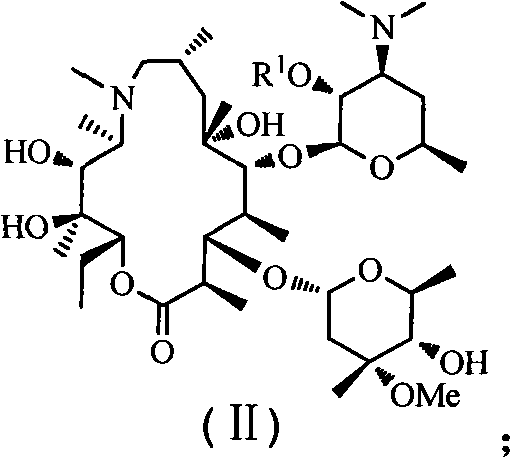
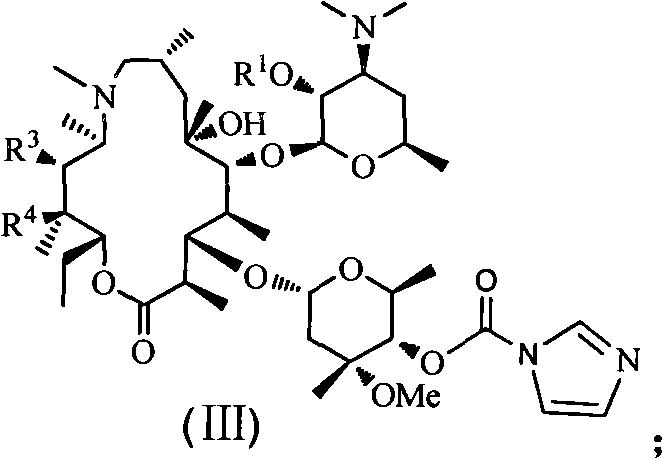
Azithromycin derivative, preparation method and intermediate thereof
A technology for azithromycin and derivatives, applied in the field of azithromycin derivatives, preparation and intermediates, which can solve problems such as side effects and increased toxicity, steric hindrance effects, and excessively long side chain lengths
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Preparation of Benzoyl Chloride
[0126] Put benzoic acid (1.00g, 8.19mmol) in a 50ml round bottom flask, add thionyl chloride (15ml, 0.20mol), heat and reflux at 80°C for 4 hours, evaporate the thionyl chloride under reduced pressure, and proceed to the next step reaction.
Embodiment 2
[0128] Preparation of Benzoylpiperazine
[0129] Put piperazine (0.7g, 8.19mmol) in a 50ml three-neck flask, add 16ml of water to dissolve it; add 4 drops of methyl orange indicator, and adjust the pH with 2mol / L hydrochloric acid solution to make it just change from yellow to red Add 16ml of acetone, slowly add benzoyl chloride dropwise under stirring in an oil bath at 50°C, and continuously neutralize the generated HCl with 40% sodium acetate solution, so that the pH of the reaction solution remains within the discoloration range of methyl orange; react for 30 Minutes, acetone was evaporated under reduced pressure, pH=12 was adjusted with 40% sodium hydroxide solution, extracted with dichloromethane, dried over anhydrous sodium sulfate, and 0.72 g of a colorless oily substance was evaporated to dryness under pressure. Yield 45.2%, Rf It was 0.65 (the developing solvent was dichloromethane:methanol=5:1).
Embodiment 3
[0131] Preparation of 4-(3-aminopropyl)piperazin-1-ylbenzophenone
[0132] Benzoylpiperazine (0.72, 3.78mmol) was placed in a 100ml round-bottomed flask, dissolved in 10ml of isopropanol, chlorpromamine hydrochloride (0.70g, 5.67mmol) and triethylamine (1.06ml, 6.56mmol) were added ), stirred in an oil bath at 50°C for 24h, heated to reflux at 90°C for 24h; evaporated to dryness under pressure, dissolved in water, adjusted to PH=12 with 40% NaOH, extracted with dichloromethane, washed with saturated sodium chloride, dried over anhydrous sodium sulfate, and reduced 0.7 g of the oily matter distilled under high pressure was subjected to silica gel column chromatography (eluent: dichloromethane: methanol = 20: 1) to obtain a colorless oily matter, which was the target compound A1 (0.3 g, 1.21 mmol), and the yield was 45.2 g. %,R f is 0.16 (the developing solvent is dichloromethane:methanol=5:1).
[0133] Other compounds with similar structures were prepared using the same metho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com