Clathrate of azithromycin hydrate with 1,2-propyleneglycol, method for the manufacture thereof and pharmaceutical composition comprising same
A technology of azithromycin and propylene glycol, applied in the field of pharmaceutical compositions comprising this inclusion compound, can solve problems such as being insufficient to remove toxic aliphatic hydrocarbon solvents, undesired water content azithromycin dihydrate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
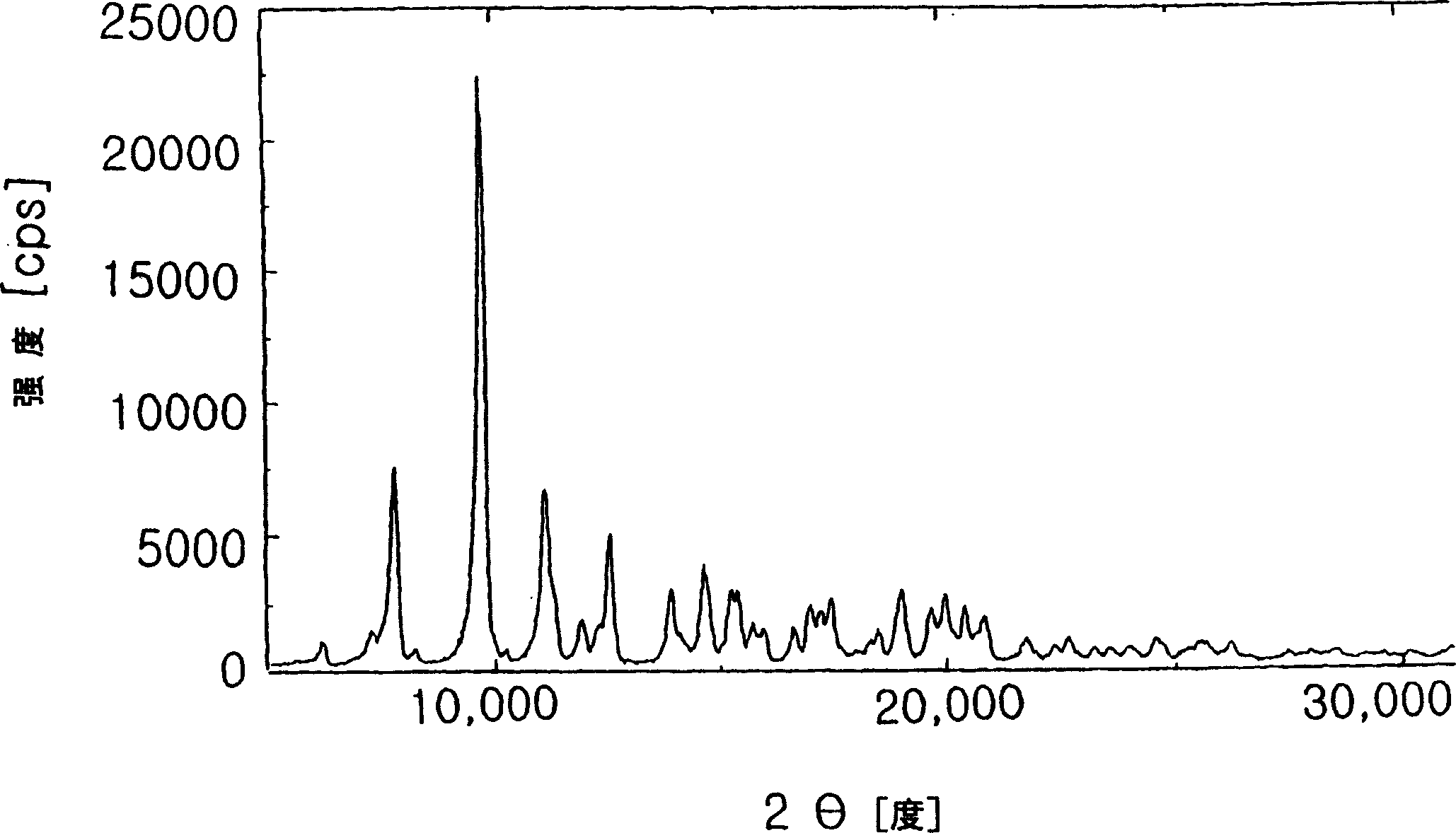
[0039] 100 g of azithromycin anhydride was dissolved in 300 ml of acetone, and 100 ml of 1,2-propanediol was added thereto. The solution was stirred at room temperature for 10 minutes, and 500 ml of water was added dropwise thereto to induce precipitation of azithromycin crystals. The solution was stirred at room temperature for 2 hours, and the precipitate was filtered, washed strictly with water, and then dried at 40° C. for 20 hours to obtain 96 g of an inclusion compound of azithromycin hydrate and 1,2-propanediol.
[0040] m.p.: 129~131℃,
[0041] Moisture content measured by Karl Fischer water analyzer: 3.5wt%,
[0042] 1,2-Propanediol content determined by gas chromatography: 3.3% by weight.
Embodiment 2
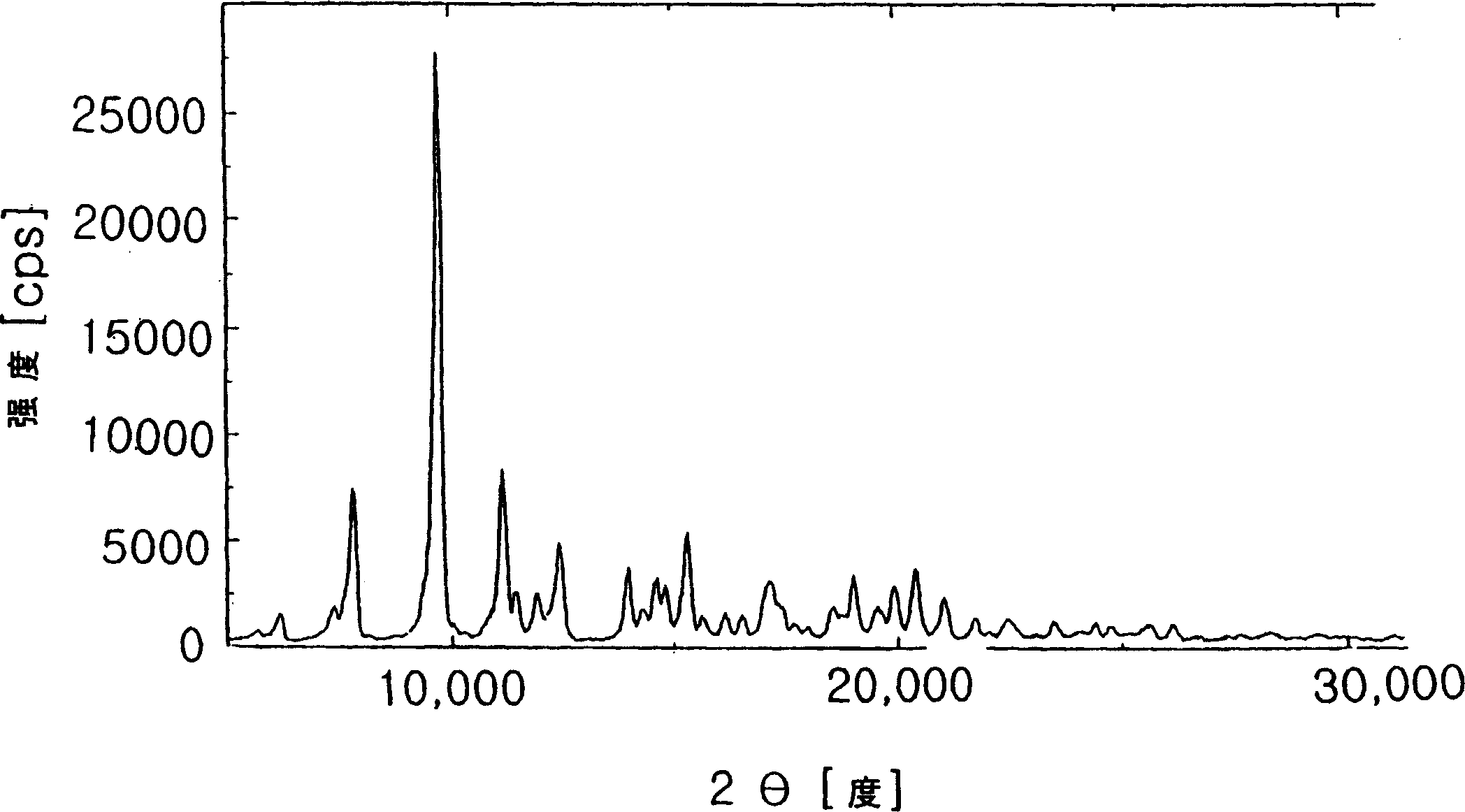
[0044] 20 g of azithromycin monohydrate was dissolved in 100 ml of acetone, and 15 ml of 1,2-propanediol was added thereto. The solution was stirred at room temperature for 10 minutes, and 200 ml of water was added dropwise thereto to induce precipitation of azithromycin crystals. The solution was stirred at 0-5° C. for 2 hours, and the precipitate was filtered, washed strictly with water, and then dried at 40° C. for 20 hours to obtain 18.2 g of an inclusion compound of azithromycin hydrate and 1,2-propanediol.
[0045] m.p.: 130~132℃,
[0046] Moisture content: 3.4wt%,
[0047] 1,2-propanediol content: 3.2 wt%.
Embodiment 3
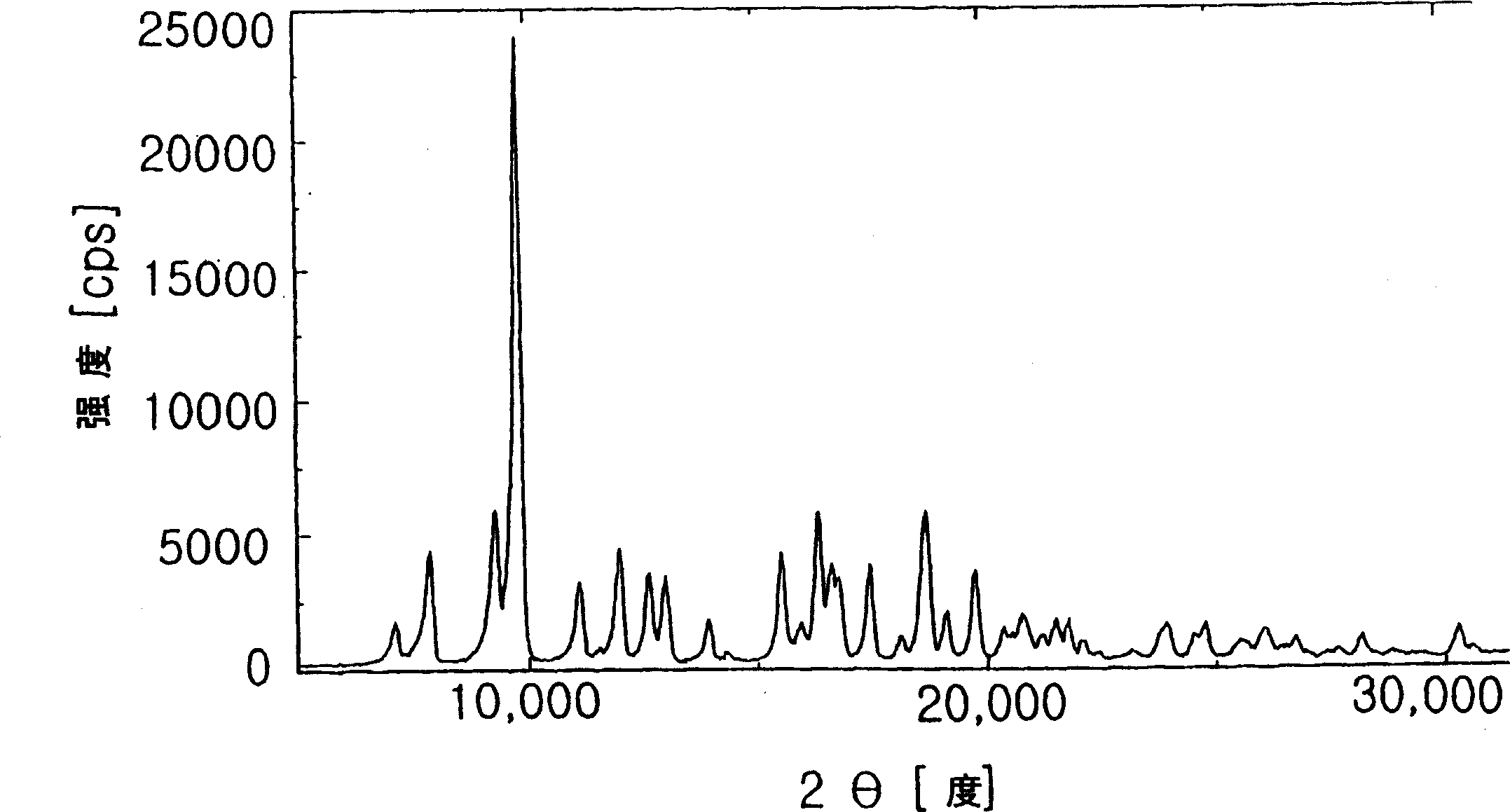
[0049]20 g of azithromycin monohydrate was dissolved in 120 ml of acetone, and 15 ml of 1,2-propanediol was added thereto. The solution was stirred at room temperature for 10 minutes, and 180 ml of water was added dropwise thereto to induce precipitation of azithromycin crystals. The solution was stirred at 0-5° C. for 3 hours, and the precipitate was filtered, washed strictly with water, and then dried at 40° C. for 20 hours to obtain 17.6 g of an inclusion compound of azithromycin hydrate and 1,2-propanediol.
[0050] m.p.: 130~132℃,
[0051] Moisture content: 3.4wt%,
[0052] 1,2-propanediol content: 3.5 wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com