Azithromycin eye drops and preparation method thereof
A technology for azithromycin and eye drops, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of prolonging the action time of the drug, difficult to control the dosage, and difficult to produce, and achieve a good biological phase. The effect of reducing the frequency of administration, improving the compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
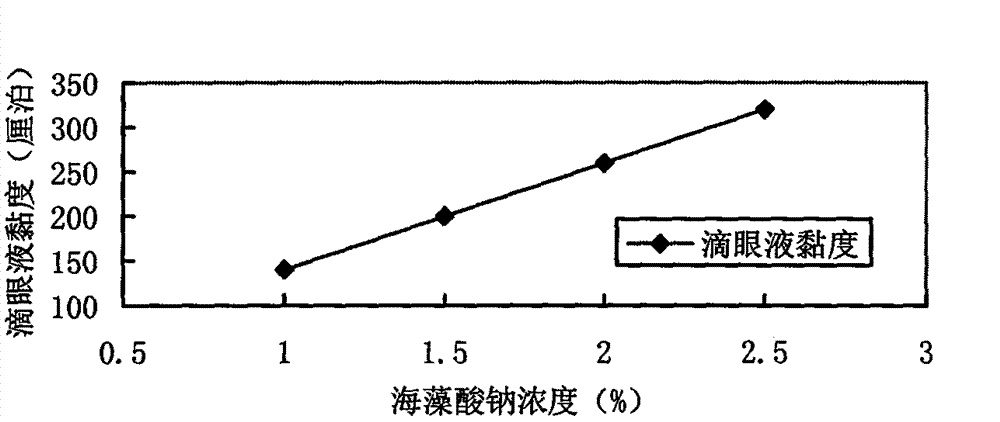
[0024] Prescription: Azithromycin: 1g Sodium Alginate: 1.5g
[0025] Citric acid: 0.2g Sodium citrate: 0.3g
[0026] EDTA-2Na: 0.05g Sodium hydroxide: appropriate amount
[0027] Benzalkonium bromide: 0.01g water for injection to 100ml
[0028] Preparation method:
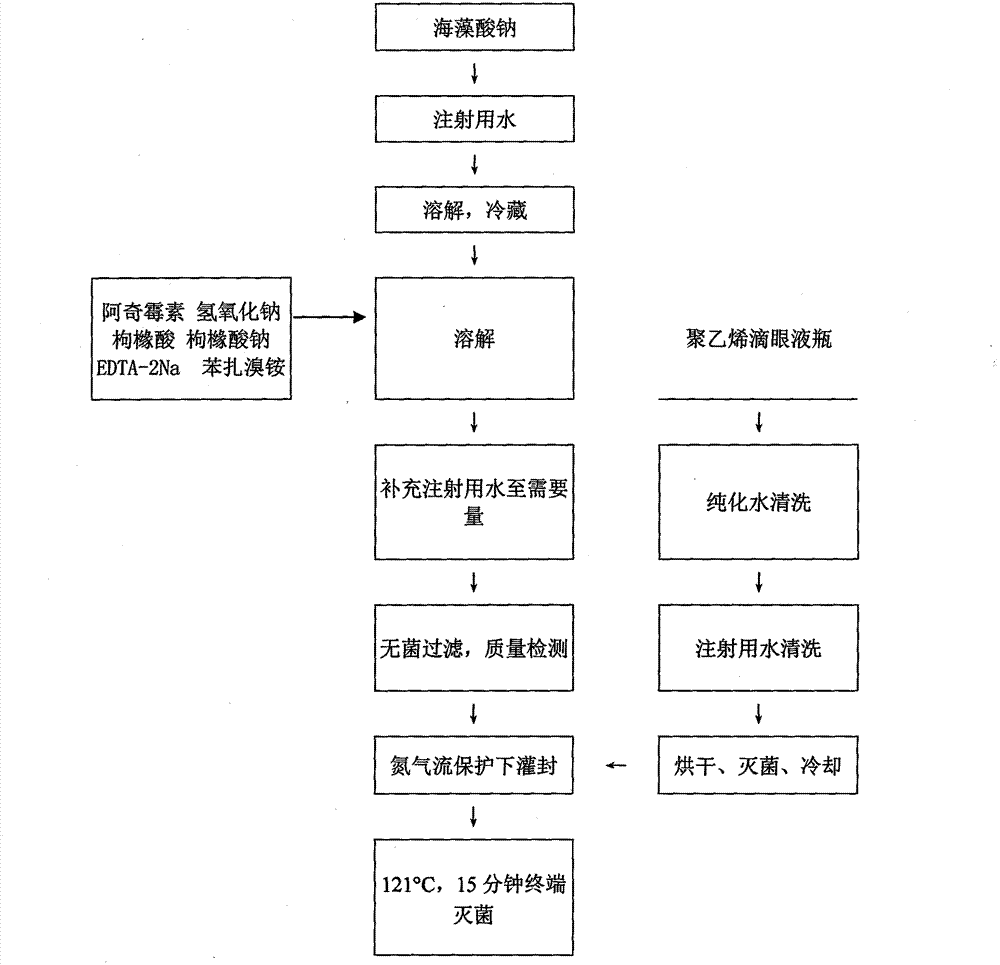
[0029] (1) Weigh sodium alginate and place it in water for injection, stir until sodium alginate completely dissolves into a solution, and refrigerate;
[0030] (2) Take azithromycin, add a pH regulator and stir until the medicine is completely dissolved, add an osmotic pressure regulator, a stabilizer, a bacteriostat, and stir evenly;
[0031] (3) Mix the solutions of steps (1) and (2), stir evenly, and replenish to the required amount with an appropriate amount of water for injection;
[0032] (4) Aseptically subpackage after sterile filtration.
Embodiment 2
[0034] Prescription: Azithromycin: 1g Sodium Alginate: 2g
[0035] Citric acid: 0.2g Sodium citrate: 0.3g
[0036] EDTA-2Na: 0.10g Sodium hydroxide: appropriate amount
[0037] Benzalkonium bromide: 0.005g
[0038] Preparation method:
[0039] (1) Weigh sodium alginate and place it in water for injection, stir until sodium alginate completely dissolves into a solution, and refrigerate;
[0040] (2) Take azithromycin, add a pH regulator and stir until the medicine is completely dissolved, add an osmotic pressure regulator, a stabilizer, a bacteriostat, and stir evenly;
[0041] (3) Mix the solutions of steps (1) and (2), stir evenly, and replenish to the required amount with an appropriate amount of water for injection;
[0042] (4) Aseptically subpackage after sterile filtration.
Embodiment 3
[0044] Prescription: Azithromycin: 1g Sodium Alginate: 1.8g
[0045] Citric acid: 0.2g Sodium citrate: 0.3g
[0046] EDTA-2Na: 0.15g Sodium hydroxide: appropriate amount
[0047] Benzalkonium bromide: 0.001g
[0048] Preparation method:
[0049] (1) Weigh sodium alginate and place it in water for injection, stir until sodium alginate completely dissolves into a solution, and refrigerate;
[0050] (2) Take azithromycin, add a pH regulator and stir until the medicine is completely dissolved, add an osmotic pressure regulator, a stabilizer, a bacteriostat, and stir evenly;
[0051] (3) Mix the solutions of steps (1) and (2), stir evenly, and replenish to the required amount with an appropriate amount of water for injection;
[0052] (4) Aseptically subpackage after sterile filtration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com