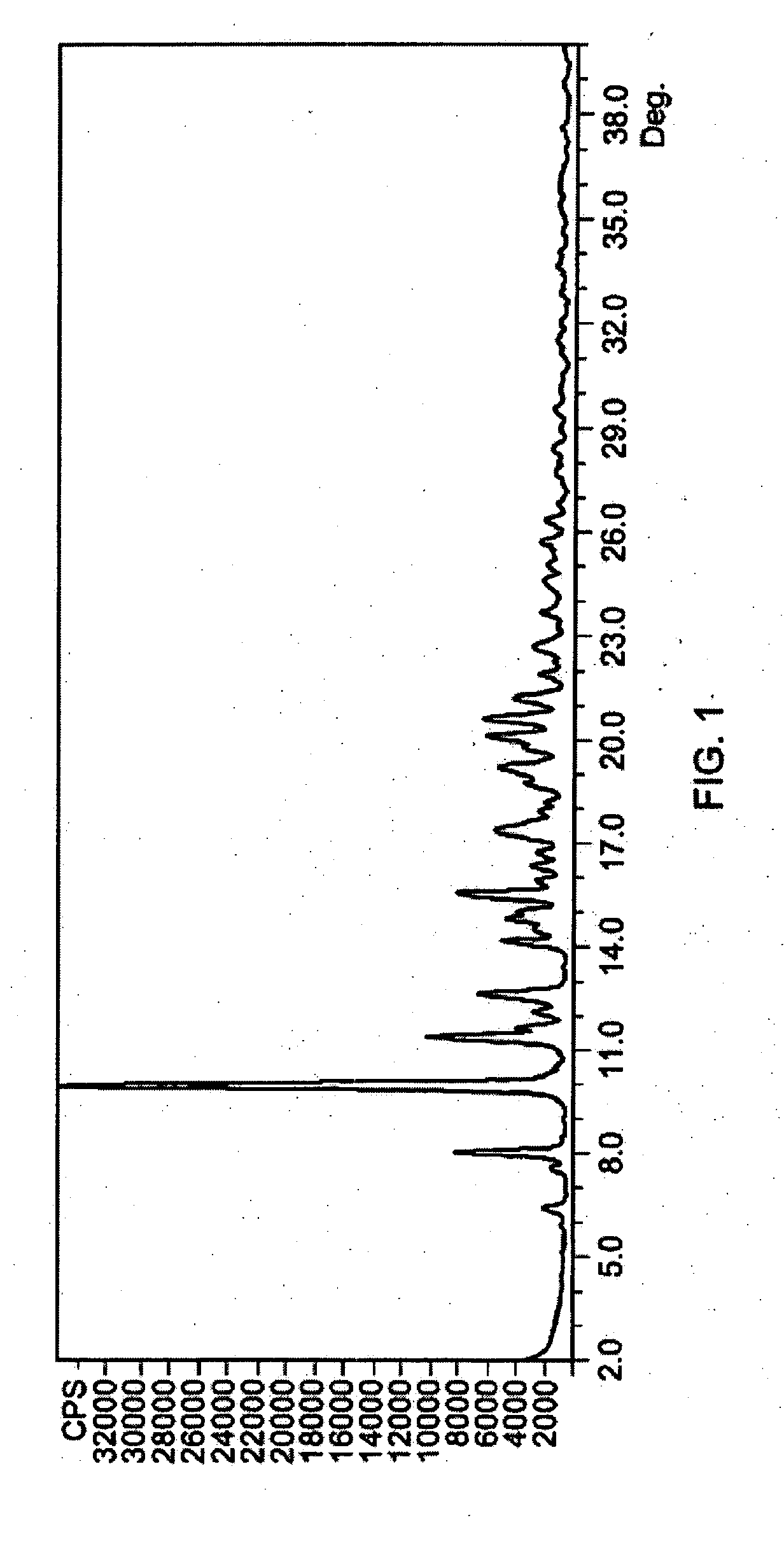
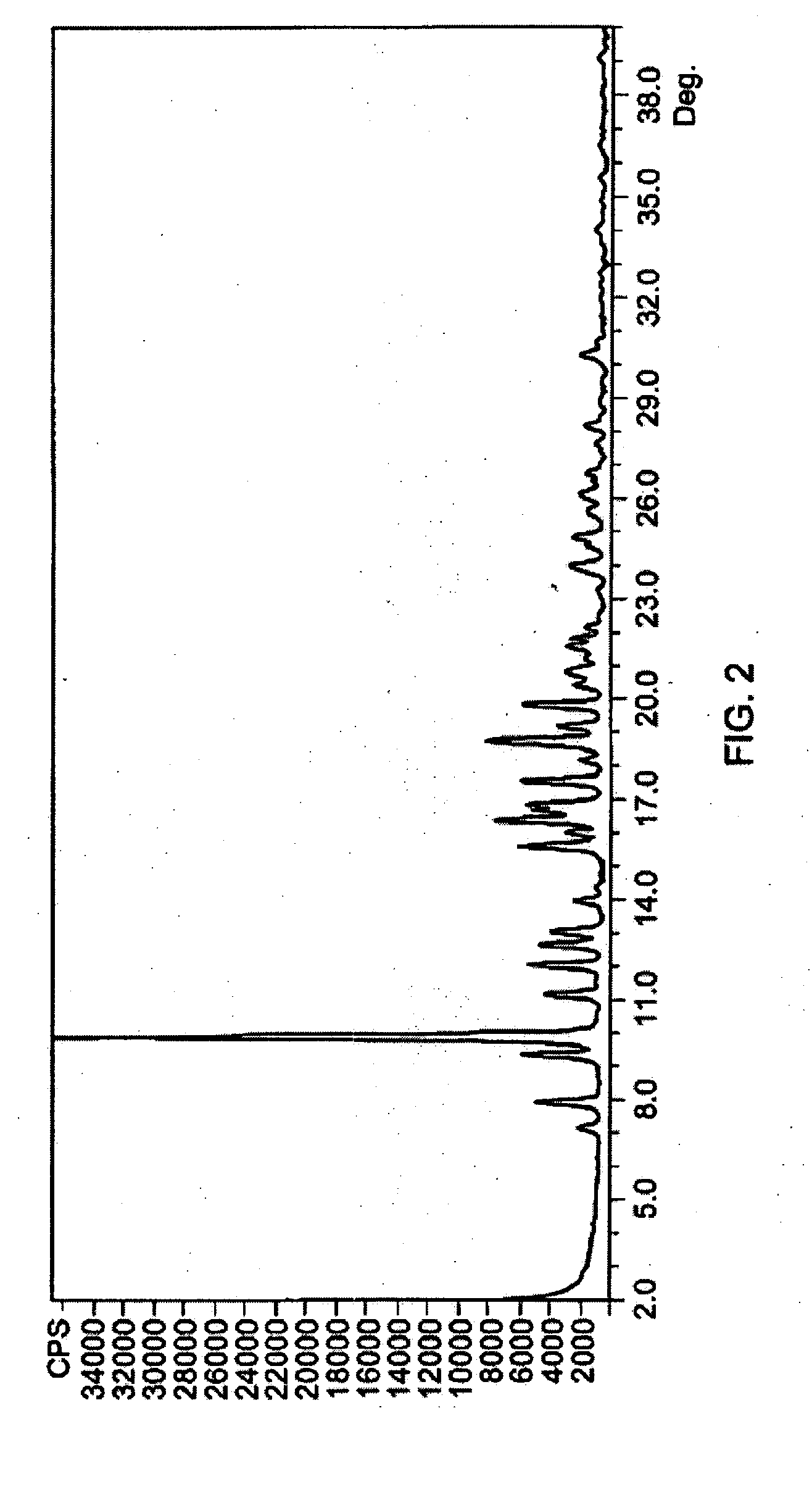
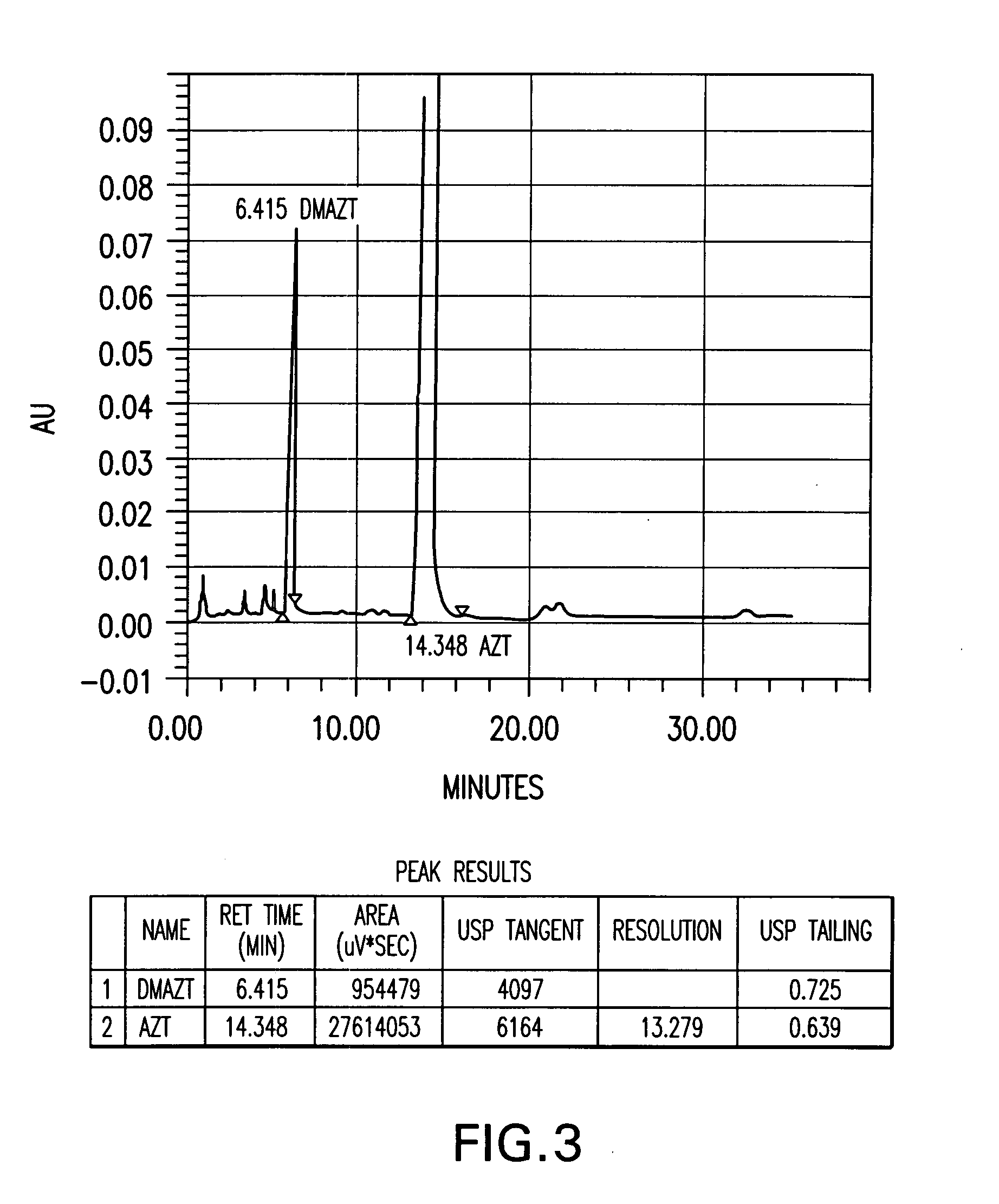
Methods of stabilizing azithromycin
a stabilizing method and azithromycin technology, applied in the field of packaging azithromycin, can solve the problems of erythromycin a, is susceptible to degradation, is unstable, etc., and achieves the effect of improving the stability of azithromycin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0073] Several azithromycin samples were analyzed using HPLC to determine the level of impurities within each sample. The analytical conditions of the HPLC were column of 150×4.6 mm; packing material of Kromasil KR 100-5C18, 5Φ; and an eluent of 40% 0.05 M K2HPO4 adjusted to a pH of 8.2 and 60% acetonitrile. The flow rate was 0.9 ml / min; the detector set at 210 nm; and column temperature of 30° C. The samples were injected into the HPLC and run for over 35 min. The impurities were determined by their relative retention times (RRT) as compared to azithromycin and were reported as a weight percent (versus azithromycin) of the total composition. Additional impurities found in the samples were reported under “other RRT” as a weight percent of the azithromycin content. The results of the analytical tests is summarized in Table A. Table A demonstrates a finding of the main azithromycin degradation products where azithromycin batches have been stored under uncontrolled temperature conditio...
example 2
Storage Testing
[0074] Three samples of azithromycin were separately packaged in a standard polyethylene bag, and then the polyethylene bags containing azithromycin were separately packaged into aluminum bags with silica gel. The stored azithromycin was submitted to stability programs either long term or accelerated to determine the effect upon azithromycin stability and the production of degradation products. The longer term stability program comprised submitting the sample to a temperature of about 25° C.±2° C. at a relative humidity of 60%±5%. The accelerated program comprised submitting the sample to a temperature of about 40° C.±2° C. at a relative humidity of 75%+5%. The samples were analyzed at regular intervals to determine the impurity profiles as assayed by HPLC using the technique described in Example 1. The water content was determined by Karl Fischer methodology; and the ethanol content was determined by gas chromatography. The results of these tests are summarized in T...
example 3
Azithromycin Stability as a Function of Storage Temperature
[0076] Samples of azithromycin were placed in storage bags and each batch sample was analyzed after storage at a variety of temperatures using the analytical techniques as described in Example 1. Each batch was packaged in a polyethylene bag and subsequently, each bag was packaged in an aluminum bag with silica gel. Table D summarizes the effects of storage temperature on the production of azithromycin degradation products. The results demonstrate that storing azithromycin at low temperatures (+5° C.) leads to inhibition of the production of degradation products.
TABLE DAzithromycin Stability as a Function of Storage TemperatureAZTTimeRRT (%)OtherTotalBatch(months)T° C.0.260.340.370.80RRT %%Batch00.070.030.1No. 432-80.070.120.060.060.120.33250.360.410.260.320.411.5Batch00.070.030.1No. 532-80.100.150.070.080.150.43250.440.620.390.430.621.9Batch00.130.070.040.130.2No. 632-80.070.170.110.030.170.43250.390.570.320.340.571.8
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com