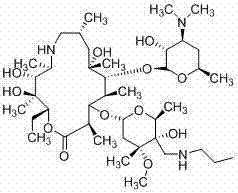
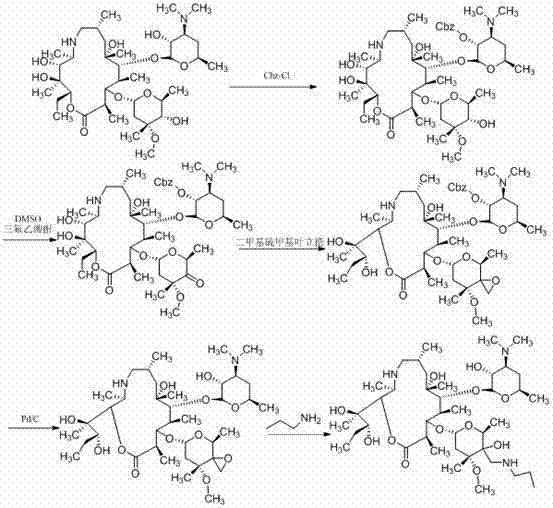
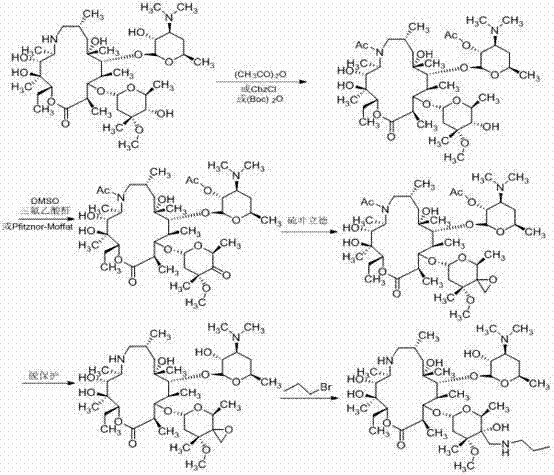
Preparation method of animal antibiotic tulathromycin
A technology for teramycin and antibiotics, which is applied in the field of preparation of teramycin and the synthesis of medicines, can solve the problems of serious environmental pollution, difficult product purification and treatment, difficult reaction control, etc., and achieves reduction of production cost and simple reaction operation. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Add oxycyclone C (0.65 g) and 15 ml methanol to a 50 ml one-necked bottle, then add triethylamine and nitromethane (0.22 ml). Heat to reflux, react for 48 h, and monitor the reaction by TLC plate. After the reaction was completed, the system was concentrated, and dichloromethane was added, and the organic layer was successively washed with NaHSO 3 Solution, water and saturated brine were washed, the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain nitromethylene adduct D with a yield of 75%.
[0039] (2) Add nitromethylene adduct D (2 g) and 20 ml methanol to a 50 ml single-necked bottle, and reduce with sodium hydrosulfite (4 g). After the reaction is completed, concentrate the system and add dichloromethane to dissolve it. Dry over sodium sulfate and evaporate the solvent to obtain protected methyleneamine E with a yield of 81%.
[0040] (3) Add protected methyleneamine E (0.7 g) and 25 ml methanol to a 50 ...
Embodiment 2
[0044] (1) Add Oxycyclone C (1.3 g) and 25 ml methanol into a 50 ml one-necked bottle, then add triethylamine and nitromethane (0.45 ml), heat to reflux, react for 48 h, and monitor the reaction by TLC plate. After the reaction was completed, the system was concentrated, and dichloromethane was added, and the organic layer was successively washed with NaHSO 3 Solution, water and saturated brine were washed, the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain nitromethylene adduct D with a yield of 78%.
[0045] (2) Add nitromethylene adduct D (4 g) and 30 ml methanol into a 50 ml single-necked bottle, and reduce it with Raney nickel hydrazine hydrate. After the reaction is completed, concentrate under reduced pressure, and then dichloromethane Extraction, drying over anhydrous sodium sulfate, evaporation of the solvent to obtain protected methyleneamine E, yield 84%.
[0046] (3) Add protected methyleneamine E (1.4 g) a...
Embodiment 3
[0050] (1) Add Oxycyclone C (3.25 g) and 50 ml methanol into a 100 ml single-necked bottle, then add triethylamine and nitromethane (1.2 ml), heat to reflux, react for 48 h, and monitor the reaction by TLC. After the reaction was completed, the system was concentrated, and dichloromethane was added, and the organic layer was successively washed with NaHSO 3 Solution, water and saturated brine were washed, the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain nitromethylene adduct D with a yield of 84%.
[0051] (2) Add nitromethylene adduct D (10 g) and 100 ml methanol into a 250 ml single-necked bottle, and reduce it with hydrosulfite (20 g). After the reaction is completed, concentrate the system and add dichloromethane to dissolve it. Anhydrous sodium sulfate is dried, and the methyleneamine E that the solvent is evaporated to dryness is protected, yield 87%
[0052] (3) Add protected methyleneamine E (3.5 g) and 80 ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com