Production method of sterile stable azithromycin eye drops
A technology for azithromycin and its production method, which is applied in the direction of antibacterial drugs, pharmaceutical formulations, medical preparations containing active ingredients, etc., and can solve the problem of harsh storage conditions, difficult access to eye drop enterprises, and restrictions on the application of azithromycin eye drops, etc. problem, to achieve the effect of improving stability and security
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The preparation method of the special solvent can be according to the conventional preparation method, adding the pH regulator and additives into the water for injection, dissolving and then sterilizing.
[0018] The osmotic pressure regulator can be conventional various osmotic pressure regulators used in eye drops, for example, it can be at least one of glycerol, sodium chloride, potassium chloride, boric acid, borax, mannitol or sorbitol kind.
[0019] The preservative can be conventional various preservatives used in eye drops, for example, it can be in benzalkonium chloride, benzalkonium bromide, methylparaben, ethylparaben, chlorobutanol at least one.
[0020] The surfactant can be various conventional surfactants used in eye drops, for example, it can be at least one of polysorbate-80, polysorbate-60 and polysorbate-20.
[0021] The antioxidant may be various conventional antioxidants used in eye drops, for example, may be at least one of disodium edetate, sodi...
Embodiment 1
[0027] Azithromycin freeze-dried powder formula:
[0028]
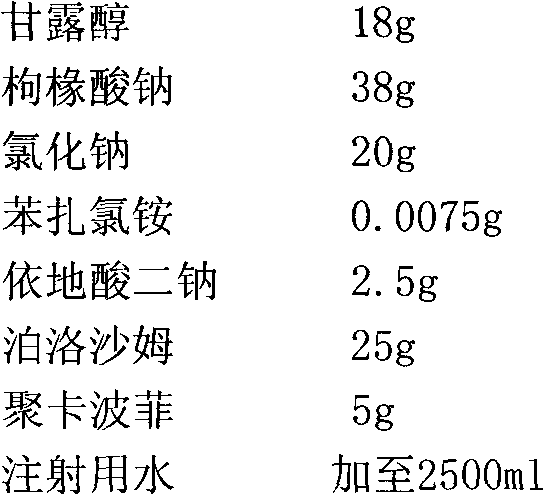
[0029] Special solvent formula:
[0030]
[0031] Preparation:
[0032] (1) Get the azithromycin and citrate of prescription quantity, join in the water for injection of prescription quantity, stir and make to dissolve, then adjust pH value to 6.0~6.5 with sodium hydroxide, 0.22 μm filter membrane sterile filtration, press each 2ml sterile aliquots, prepared into sterile freeze-dried powder according to the freeze-drying process.
[0033] (2) Take 60% of the prescription amount of water for injection, slowly add the polycarbophil of the prescription amount, swell the liquid, stir to make it even, and sterilize it by autoclaving at 121°C for 30 minutes; take another 40% of the prescription amount of water for injection, Add mannitol, sodium citrate, sodium chloride, benzalkonium chloride, disodium edetate, and poloxamer in sequence, stir to dissolve, filter aseptically with a 0.22 μm filter membrane; then mix t...
Embodiment 2
[0035] Azithromycin freeze-dried powder formula:
[0036]
[0037] Special solvent formula:
[0038]
[0039] Preparation:
[0040] (1) Get the azithromycin and citrate of prescription quantity, join in the water for injection of prescription quantity, stir and make to dissolve, then adjust pH value to 6.0~6.5 with sodium hydroxide, 0.22 μm filter membrane sterile filtration, press each 2ml sterile aliquots, prepared into sterile freeze-dried powder according to the freeze-drying process.
[0041] (2) Take 60% of the prescription amount of water for injection, slowly add the polycarbophil of the prescription amount, swell the liquid, stir to make it even, and sterilize it by autoclaving at 121°C for 30 minutes; take another 40% of the prescription amount of water for injection, Add mannitol, sodium citrate, sodium chloride, benzalkonium chloride, disodium edetate, polysorbate-80 successively, stir to dissolve, filter aseptically with a 0.22 μm filter membrane; then mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com